Abstract
Objective
The aim of this study was to evaluate the impact of para-aortic lymphadenectomy up to the renal vessels on the accurate staging in ovarian cancer patients presumed preoperatively to be confined to the ovary.
Methods
We retrospectively analyzed data on 124 patients with primary epithelial ovarian cancer who were preoperatively thought to have tumor confined to the ovary and underwent primary staging surgery. The distribution of lymph node metastasis and various risk factors for nodal involvement were investigated.
Results
Surgical staging yielded: 87 (70.2%) patients had International Federation of Gynecology and Obstetrics (FIGO) stage I disease and 37 (29.8%) patients had stage II-III disease: 4 IIA, 6 IIB, 9 IIC, 1 IIIA, and 17 IIIC. Eighty-six patients had pelvic lymphadenectomy only and 69 had pelvic and para-aortic lymphadenectomy. Lymph node metastases were found in 17 (24.6%) of 69 patients; 5 (7.2%) patients had lymph node metastasis in the pelvic lymph nodes only, 8 (11.6%) in the para-aortic lymph nodes only, and 4 (5.8%) in both pelvic and para-aortic lymph nodes. Six (8.7%) patients had lymph node metastasis in the para-aortic lymph node above the level of the inferior mesenteric artery. On multivariate analysis, grade 3 tumor (p=0.01) and positive cytology (p=0.03) were independent predictors for lymph node metastasis.
Conclusion
A substantial number of patients with apparently early ovarian cancer had upstaged disease. Of patients who underwent lymphadenectomy, some patients had lymph node metastasis above the level of the inferior mesenteric artery. Para-aortic lymphadenectomy up to the renal vessels may detect occult metastasis and be of help in tailoring appropriate adjuvant treatment as well as giving useful information about the prognosis.
Ovarian cancer remains one of the major causes of death from the female genital tract malignancy worldwide, and in the United States, 21,990 new cases and 15,460 deaths were estimated in 2011 [1]. Approximately 25% of ovarian cancer patients are diagnosed with early-stage disease at the time of initial treatment [2]. Surgical staging is a critical aspect of early ovarian cancer as well as advanced ovarian cancer because the International Federation of Gynecology and Obstetrics (FIGO) staging based on surgical and pathologic findings is one of the most important prognostic factors [3]. Accurate surgical staging for early-stage ovarian cancer patients has great significance, permitting accurate estimation of the true extent of disease with detection of occult disease, and providing patients with appropriate information about the prognosis and adjuvant treatment. Up to 30% of patients with apparent early-stage ovarian cancer are found to have extrapelvic involvement after comprehensive surgical staging [4,5]. However, all patients with early ovarian cancer do not have complete surgical staging. Approximately 33-67% of patients with this disease are inadequately staged and much of this is attributed to the insufficient evaluation of pelvic and para-aortic lymph nodes, although lymphadenectomy is an integral part of surgical staging [6].
The incidence of lymph node metastasis in patients with ovarian cancer presumed to be confined to the ovary has been reported to be 10% to 25% [7-11]. Metastasis to the para-aortic lymph nodes is the primary route of lymphatic dissemination in ovarian cancer, and the high para-aortic lymph node above the inferior mesenteric artery (IMA) is a frequently involved site [12-16]. Despite this, lymphadenectomy has not been performed in practice as a part of the routine staging procedure. Two recent retrospective analyses of the Surveillance, Epidemiology and End Results (SEER) and the Centers for Disease Control and Prevention's National Program of Cancer Registries (CDC-NPCR) data showed that lymphadenectomy was omitted in 28% to 40% of early-stage ovarian cancer patients [17,18]. To date, the extent of lymphadenectomy in early ovarian cancer is an issue under debate. The contemporary FIGO guidelines for ovarian cancer recommend pelvic and para-aortic lymphadenectomy as part of initial surgical staging procedure but do not provide the extent of lymphadenectomy [19], although several studies have addressed the potential risk of para-aortic lymph node metastasis above the level of the IMA in apparent early ovarian cancer [7,10,13,14,16,20,21]. The purpose of this study was to evaluate the incidence of pelvic and para-aortic lymph node metastasis, to identify the potential risk of para-aortic lymph node metastasis above the level of the inferior mesenteric artery in patients with ovarian cancer presumed preoperatively to be confined to the ovary, and to assess the clinical relevance of lymphadenectomy as part of the surgical staging procedure.
The medical records of all patients with ovarian cancer treated at Ajou University Hospital from January 1, 2000 through December 31, 2011 were retrospectively reviewed. Women with ovarian cancer that was thought to be confined to the ovary without any extraovarian metastatic lesions at the time of preoperative imaging studies such as computed tomography (CT) or magnetic resonance imaging (MRI) were included in the study.
All patients were surgically staged according to the FIGO system. Standard surgical staging procedures included total abdominal hysterectomy (TAH), unilateral salpingo-oophorectomy (USO) or bilateral salpingo-oophorectomy (BSO), peritoneal washings for cytology, infracolic omentectomy, multiple biopsies of pelvic and abdominal peritoneum, pelvic lymphadenectomy, and para-aortic lymphadenectomy. Pelvic lymphadenectomy included bilateral resection of the common iliac nodes, presacral nodes, external iliac nodes, internal iliac nodes, deep inguinal nodes, and obturator nodes. Para-aortic lymphadenectomy included removal of all nodal tissues over the vena cava and aorta from the aortic bifurcation to the level of the renal vessels. With regard to anatomic distributions of resected lymph nodes, all pelvic lymph nodes were separately sent to pathology with dividing into the right and left pelvic lymph nodes. Most of the para-aortic lymph nodes were separately sent to the intraoperative frozen section or postoperative permanent section for pathologic evaluation with dividing into low and high para-aortic lymph nodes according to the IMA. However, para-aortic lymph nodes from some patients were sent en bloc to pathology without dividing into low and high nodes.
Some patients did not undergo complete staging procedures if all of the following conditions were present: patients whose preoperative and intraoperative findings were no gross lesions on the contralateral ovary, uterus, and other pelvic and abdominal organs; and patients who were found to have no grossly enlarged lymph nodes in the retroperitoneal area by preoperative imaging studies, including CT, and by intraoperative inspection and palpation by the operating surgeon. Young, unmarried women who met the above conditions and who wanted to preserve her fertility did not undergo hysterectomy and contralateral salpingo-oophorectomy. All surgical procedures were performed by five gynecologic oncologists, and the decision as to which surgical procedures would be performed was based on the discretion of the operating surgeon. Postoperatively, all patients except those with FIGO stage IA or IB, grade 1 or 2 disease received taxane-/platinum-based systemic chemotherapy (paclitaxel plus cisplatin or carboplatin) for 3-6 cycles at 3-week intervals regardless of performing lymphadenectomy. However, patients with stage IA or IB, grade 2 disease who did not undergo lymphadenectomy received adjuvant chemotherapy.
Patients were classified into two groups: patients who underwent systematic pelvic and para-aortic lymphadenectomy and those who did not. Some patients underwent only minimal lymph node sampling and these were included in the group without systematic lymphadenectomy. Information regarding demographic data, preoperative evaluations, intraoperative findings, pathologic features, and follow-up was abstracted from medical records, and these were compared between both groups. SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of observed data. The chi-square or Fisher's exact test were used for comparison of observed frequencies. Student t-test or Mann-Whitney U-test were applied for comparison of continuous variables. Progression-free survival was defined as the time from primary surgery to disease recurrence. Overall survival was defined as the time from primary surgery to death. Kaplan-Meier method was used to estimate progression-free and overall survival rates of patients with and without lymphadenectomy, and the log-rank test was used to compare survival functions. A logistic regression model was performed for multivariate analysis and used in estimating the odds ratios of various parameters which were found to be significant in the univariate analysis. Backward stepwise model-selection methods, using a cutoff p-value of 0.05, were used to select factors that were included in the multivariate analysis. Statistical significance was defined as p<0.05.
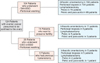
A total of 124 consecutive patients were identified during this time period. The median age was 46 years (range, 19 to 74 years). Surgical staging procedures are schematized in Fig. 1. One hundred and four (83.9%) patients received TAH. Twenty (16.1%) patients did not receive TAH because of previous hysterectomy (5 patients) and planning fertility-preservation (15 patients). Peritoneal washings, infracolic omentectomy, and multiple peritoneal biopsies were performed in 124 (100%), 120 (96.8%), and 117 (94.4%) patients, respectively. Pelvic and para-aortic lymphadenectomy were performed in 86 (69.4%) and 69 (55.6%) patients, respectively. Nine patients underwent only lymph node sampling or biopsy-external iliac node sampling in 4 patients and both external and internal iliac node sampling in 5 patients. The mean operative time was significantly longer in patients with systematic lymphadenectomy compared to those without lymphadenectomy (220 minutes vs. 98 minutes, p<0.01). During surgery, 3 patients had grossly enlarged (larger than 1 cm in diameter on intraoperative palpation by the surgeon) para-aortic lymph node and all of them underwent pelvic and para-aortic lymphadenectomy.
Overall, 87 (70.2%) patients were found to have FIGO stage I disease: 54 IA, 2 IB, and 31 IC. Nineteen (15.3%) patients had stage II disease: 4 IIA, 6 IIB, and 9 IIC and 18 (14.5%) had stage III disease: 1 IIIA and 17 IIIC. Ascites was present in 34.7% of patients.
Patients with systematic lymphadenectomy had higher parity (p<0.01), more frequent ascites (p=0.01), and higher numbers of resected lymph nodes (p<0.01). There were no statistically significant differences in demographic features, FIGO stage, tumor histology, tumor grade, and preoperative CA-125 level between the two groups (Table 1). Adjuvant chemotherapy was given to 78 (62.9%) patients and the proportion of patients receiving adjuvant chemotherapy was significantly higher in the lymphadenectomy group (70.9% vs. 44.7%, p<0.01). Among patients with lymphadenectomy, 5 had lymphocysts, which were successfully treated by conservative measures.
Table 2 demonstrates the distribution of lymph node metastasis. Of 69 patients who underwent para-aortic lymphadenectomy, lymph node metastases were found in 17 (24.6%) patients: 5 (7.2%) patients had lymph node metastasis in the pelvic lymph nodes only, 8 (11.6%) in the para-aortic lymph nodes only, and 9 (13.0%) in both pelvic and para-aortic lymph nodes. Six (8.7%) patients had para-aortic nodal involvement above the level of the IMA.
Serous histology, grade 3 tumors, presence of ascites, and positive peritoneal cytology were found to be significant prognostic factors on univariate analysis for lymph node metastasis. On multivariate analysis, grade 3 tumor (odds ratio [OR], 5.42; 95% confidence interval [CI], 1.51-19.52; p=0.01) and positive cytology (OR, 4.22; 95% CI, 1.12-15.96; p=0.03) were independent predictors for lymph node metastasis (Table 3).
In the entire cohort of 124 patients, the 5-year progression-free and overall survival rates were 68% and 77% in the no lymphadenectomy group and 71% and 89% in the lymphadenectomy group, respectively. There were no significant differences in progression-free (p=0.49) and overall survival (p=0.35) between the two groups (Fig. 2).
In 1983, Young et al. [4] provided a plausible account of the significance of comprehensive surgical staging for clinically early-stage ovarian cancer. These investigators performed systematic restaging laparotomy for 100 patients referred to them with early ovarian cancer. Thirty-one percent of patients were found to have upstaged disease and 77% of these patients had stage III disease. The most frequent sites of extrapelvic involvement were peritoneal washings (19%) and para-aortic nodes (19%). In 2012, a Group of European Investigators reported their institutional series on the importance of surgical staging in early ovarian cancer patients [22]. Grabowski et al. [22] restaged 35 patients referred to their institution with presumed early ovarian cancer limited to the pelvis. After comprehensive restaging surgery, 50% of patients were upstaged and 65% of upstaged patients had stage IIIC disease. Pelvic peritoneum (34%) and para-aortic lymph nodes (32%) were the most commonly involved sites. It is very interesting that the pattern of surgical practice for early ovarian cancer has not changed for three decades. From these studies, some findings can be drawn: 1) up to now not all patients with apparent early ovarian cancer undergo comprehensive surgical staging procedures, and 2) a considerable number of upstaged patients have stage III disease and para-aortic lymph node is one of commonly involved sites. The present study takes it as the focal point of para-aortic lymph node metastasis in early ovarian cancer.
In the present study, 24.6% of apparent early ovarian cancer patients who underwent pelvic and para-aortic lymphadenectomy had lymph node metastasis on postoperative findings, although preoperative CT or MRI performed in all patients did not reveal suspicious nodal metastases. Of these node-positive patients, 47% had para-aortic node metastasis only. These findings are consistent with the data of other studies reporting that up to 30% of early ovarian cancer patients have positive lymph nodes and 50% of patients with positive nodes have para-aortic nodal involvement only [6-8,10,11,13,14,16,20,21].
With regard to the location of para-aortic nodal disease, 8.7% of patients in the present study who underwent pelvic and para-aortic lymphadenectomy had para-aortic nodal involvement above the level of the IMA. Several investigators have reported series on the incidence and distribution of para-aortic lymph node metastasis in early ovarian cancer, but relatively few studies have attempted to evaluate the para-aortic nodal involvement above the IMA [7-11,13,14,16,20,23] (Table 4). Most of these studies do not provide the accurate information on para-aortic lymph node metastasis above the IMA in patients with presumed ovarian cancer confined to the ovary because of analyzing data including patients with stage II disease. Onda et al. [13] retrospectively reviewed 110 epithelial ovarian cancer patients who underwent systematic pelvic and para-aortic lymphadenectomy. They identified 59 patients with clinical stage I-II disease and found that 13 (22.0%) had lymph node metastases. Nine patients (15.3%) had para-aortic nodal disease above the inferior mesenteric artery, and the authors suggested that para-aortic lymph nodes above the IMA should be biopsied routinely in staging ovarian cancer. However, other investigators reported that the incidence rate of para-aortic lymph node metastasis above the IMA ranged from 4.3% to 8.6% [7,11,14,20].
In the light of these considerations, in order to adequately surgically assess patients with suspected early-stage ovarian cancer, it is important to thoroughly evaluate retroperitoneal lymph nodes, and complete para-aortic lymphadenectomy up to the renal vessels should be performed as an integral part of surgical staging procedure even in early-stage disease. However, lymphadenectomy is not routinely performed at the initial staging surgery. In our study, pelvic or para-aortic lymphadenectomy were performed in approximately 70% of patients and comprehensive surgical staging including pelvic and para-aortic lymphadenectomy was performed in 60% of patients. These findings were similar to others. In 2006, Goff et al. [24] analyzed the surgical data of 10,432 women using hospital records from nine states and described the patterns of surgical care in the United States. There were 4,057 patients with early ovarian cancer and 53.1% of patients had lymph node biopsy or dissection. Recently, Chan et al. [17] and Cress et al. [18] conducted a retrospective analysis of the SEER data of 8,372 patients and the CDC-NPCR data of 721 patients, respectively. All patients presented with early-stage disease and lymphadenectomy was performed in 60% and 70% of patients, respectively.
The low rates of performing lymphadenectomy seem to be due to the fact that so far, no definitive answer has been given to the therapeutic benefit of lymphadenectomy. In 2006, Maggioni et al. [23] published the results of randomized study on the value of systematic lymphadenectomy for early ovarian cancer. These investigators randomly assigned 268 patients to the lymphadenectomy group (n=138) and the lymph node sampling group (n=130). There was no statistically significant difference in survival between the two groups, but the median operating time was longer and the morbidity was higher in the lymphadenectomy group. Despite its prospective nature, the study may have lacked power to determine a survival impact of lymphadenectomy because of the small number of patients and inconsistent adjuvant chemotherapy to each group. Like the Maggioni et al. [23] study, the present study showed that the progression-free and overall survival of patients undergoing lymphadenectomy did not differ from those of patients who had not lymphadenectomy. However, considering the small number of patients studied, it may be underpowered to detect a distinct survival difference between the two groups. Several retrospective studies have shown that lymphadenectomy is associated with improved survival of patients with early ovarian cancer [6,17,25]. Timmers et al. [6] retrospectively analyzed data which were collected for the European Organization for Research and Treatment of Cancer-Adjuvant Chemotherapy in Ovarian Neoplasm (EORTC-ACTION) trial on surgical staging and adjuvant chemotherapy for early-stage ovarian cancer. In this study of 135 patients with early ovarian cancer, lymph node sampling and blind peritoneal biopsies were associated with improved progression-free and overall survival. More recently, Cress et al. [18] reviewed the medical records of 721 early ovarian cancer patients resided in California and New York using population-based cancer registries. Of surgical procedures performed, only lymphadenectomy was strongly associated with improved survival.
There are several limitations of our study. The first limitation is that the total lymph node count retrieved is relatively small. The median numbers of pelvic and para-aortic lymph nodes harvested were 25 and 10, respectively. This may result in the underestimation of the incidence of nodal metastasis in our study. The second limitation is selection bias, which is inherent in any retrospective study. Only 69.4% and 55.6% of patients underwent pelvic and para-aortic lymphadenectomy. Although the decision to perform lymphadenectomy and other staging procedures was determined by gynecologic oncologists, the difference in operating surgeons may have influence on our results. Approximately 24% of patients without lymphadenectomy underwent lymph node sampling or biopsy. Moreover, about 45% of patients without lymphadenectomy received adjuvant chemotherapy. These may affect survival outcome in our study. The third limitation is a lack of accurate para-aortic lymph node mapping. The information on anatomic distribution of resected lymph nodes was retrospectively collected based on operative records and pathologic reports. Not all para-aortic lymph nodes were sent separately to pathology for examination with accurate mapping of low and high para-aortic basin according to the IMA. Para-aortic lymph nodes from 10 (15%) patients were not divided separately into low and high para-aortic nodes. Admittedly, this could lead to the potential overestimation or underestimation of para-aortic lymph node metastasis and may limit the ability to draw clear conclusions.
Despite these limitations, our findings suggest the importance of para-aortic lymphadenectomy above the IMA in patients with apparently clinical stage I ovarian cancer. The homogenous characteristics of our study cohort consisting of clinical stage I ovarian cancer may provide more accurate information about the distribution of high para-aortic nodal metastasis because most other studies on this issue included patients presumed clinical stage II disease.
In summary, a substantial number of patients with apparently early ovarian cancer had upstaged disease. Of patients who underwent lymphadenectomy, about one-fourth of patients had lymph node metastasis, and three-fourths of patients with para-aortic nodal involvement had positive para-aortic lymph nodes above the level of the inferior mesenteric artery. Complete staging operation including para-aortic lymphadenectomy up to the renal vessels could detect occult metastasis and be of help in tailoring appropriate adjuvant treatment with avoiding unnecessary chemotherapy. This may indirectly influence survival and quality of life in these patients.
Figures and Tables
Fig. 1
Surgical procedures performed. TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; USO, unilateral salpingo-oophorectomy.
References
1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011. 61:212–236.
2. Chan JK, Munro EG, Cheung MK, Husain A, Teng NN, Berek JS, et al. Association of lymphadenectomy and survival in stage I ovarian cancer patients. Obstet Gynecol. 2007. 109:12–19.
3. Shih KK, Chi DS. Maximal cytoreductive effort in epithelial ovarian cancer surgery. J Gynecol Oncol. 2010. 21:75–80.
4. Young RC, Decker DG, Wharton JT, Piver MS, Sindelar WF, Edwards BK, et al. Staging laparotomy in early ovarian cancer. JAMA. 1983. 250:3072–3076.
5. Le T, Adolph A, Krepart GV, Lotocki R, Heywood MS. The benefits of comprehensive surgical staging in the management of early-stage epithelial ovarian carcinoma. Gynecol Oncol. 2002. 85:351–355.
6. Timmers PJ, Zwinderman K, Coens C, Vergote I, Trimbos JB. Lymph node sampling and taking of blind biopsies are important elements of the surgical staging of early ovarian cancer. Int J Gynecol Cancer. 2010. 20:1142–1147.
7. Suzuki M, Ohwada M, Yamada T, Kohno T, Sekiguchi I, Sato I. Lymph node metastasis in stage I epithelial ovarian cancer. Gynecol Oncol. 2000. 79:305–308.
8. Cass I, Li AJ, Runowicz CD, Fields AL, Goldberg GL, Leuchter RS, et al. Pattern of lymph node metastases in clinically unilateral stage I invasive epithelial ovarian carcinomas. Gynecol Oncol. 2001. 80:56–61.
9. Takeshima N, Hirai Y, Umayahara K, Fujiwara K, Takizawa K, Hasumi K. Lymph node metastasis in ovarian cancer: difference between serous and non-serous primary tumors. Gynecol Oncol. 2005. 99:427–431.
10. Powless CA, Aletti GD, Bakkum-Gamez JN, Cliby WA. Risk factors for lymph node metastasis in apparent early-stage epithelial ovarian cancer: implications for surgical staging. Gynecol Oncol. 2011. 122:536–540.
11. Nomura H, Tsuda H, Susumu N, Fujii T, Banno K, Kataoka F, et al. Lymph node metastasis in grossly apparent stages I and II epithelial ovarian cancer. Int J Gynecol Cancer. 2010. 20:341–345.
12. Burghardt E, Girardi F, Lahousen M, Tamussino K, Stettner H. Patterns of pelvic and paraaortic lymph node involvement in ovarian cancer. Gynecol Oncol. 1991. 40:103–106.
13. Onda T, Yoshikawa H, Yokota H, Yasugi T, Taketani Y. Assessment of metastases to aortic and pelvic lymph nodes in epithelial ovarian carcinoma: a proposal for essential sites for lymph node biopsy. Cancer. 1996. 78:803–808.
14. Morice P, Joulie F, Camatte S, Atallah D, Rouzier R, Pautier P, et al. Lymph node involvement in epithelial ovarian cancer: analysis of 276 pelvic and paraaortic lymphadenectomies and surgical implications. J Am Coll Surg. 2003. 197:198–205.
15. Pereira A, Magrina JF, Rey V, Cortes M, Magtibay PM. Pelvic and aortic lymph node metastasis in epithelial ovarian cancer. Gynecol Oncol. 2007. 105:604–608.
16. Harter P, Gnauert K, Hils R, Lehmann TG, Fisseler-Eckhoff A, Traut A, et al. Pattern and clinical predictors of lymph node metastases in epithelial ovarian cancer. Int J Gynecol Cancer. 2007. 17:1238–1244.
17. Chan J, Fuh K, Shin J, Cheung M, Powell C, Chen LM, et al. The treatment and outcomes of early-stage epithelial ovarian cancer: have we made any progress? Br J Cancer. 2008. 98:1191–1196.
18. Cress RD, Bauer K, O'Malley CD, Kahn AR, Schymura MJ, Wike JM, et al. Surgical staging of early stage epithelial ovarian cancer: results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol. 2011. 121:94–99.
19. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO Committee on Gynecologic Oncology. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. Int J Gynaecol Obstet. 2000. 70:209–262.
20. Tsumura N, Sakuragi N, Hareyama H, Satoh C, Oikawa M, Yamada H, et al. Distribution pattern and risk factors of pelvic and para-aortic lymph node metastasis in epithelial ovarian carcinoma. Int J Cancer. 1998. 79:526–530.
21. Sakuragi N, Yamada H, Oikawa M, Okuyama K, Fujino T, Sagawa T, et al. Prognostic significance of lymph node metastasis and clear cell histology in ovarian carcinoma limited to the pelvis (pT1M0 and pT2M0). Gynecol Oncol. 2000. 79:251–255.
22. Grabowski JP, Harter P, Buhrmann C, Lorenz D, Hils R, Kommoss S, et al. Re-operation outcome in patients referred to a gynecologic oncology center with presumed ovarian cancer FIGO I-IIIA after sub-standard initial surgery. Surg Oncol. 2012. 21:31–35.
23. Maggioni A, Benedetti Panici P, Dell'Anna T, Landoni F, Lissoni A, Pellegrino A, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer. 2006. 95:699–704.
24. Goff BA, Matthews BJ, Wynn M, Muntz HG, Lishner DM, Baldwin LM. Ovarian cancer: patterns of surgical care across the United States. Gynecol Oncol. 2006. 103:383–390.
25. Rouzier R, Bergzoll C, Brun JL, Dubernard G, Selle F, Uzan S, et al. The role of lymph node resection in ovarian cancer: analysis of the Surveillance, Epidemiology, and End Results (SEER) database. BJOG. 2010. 117:1451–1458.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download