Abstract
Objectives
To understand the philosophy of evidence-based medicine (EBM) in order to support clinical decision making.
Summary of Literature Review
Evidence-based medicine is a commonplace phrase representing the hallmark of excellence in clinical practice. However, there has been misunderstanding and indiscriminate use of the concept of EBM in clinical practice. It is necessary to understand true philosophy of EBM.
REFERENCES
1. Fisher CG, Wood KB. Introduction to and techniques of evidence-based medicine. Spine (Phila Pa 1976). 2007; 32(19 Suppl):S66–72.

2. Schü nemann HJ, Bone L. Evidence-based orthopaedics: a primer. Clin Orthop Relat Res. 2003; 413:117–32.
3. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ. 1996; 312:71–2.

5. Cherkin DC, Deyo RA, Loeser JD, Bush T, Waddell G. An international comparison of back surgery rates. Spine (Phila Pa 1976). 1994; 19:1201–6.

6. Gibson JN, Grant IC, Waddell G. The Cochrane review of surgery for lumbar disc prolapse and degenerative lumbar spondylosis. Spine (Phila Pa 1976). 1999; 24:1820–32.

7. Gartland JJ. Orthopaedic clinical research. Deficiencies in experimental design and determinations of outcome. J Bone Joint Surg Am. 1988; 70:1357–64.

9. Obremskey WT, Pappas N, Attallah-Wasif E, Tornetta P 3rd, Bhandari M. Level of evidence in orthopaedic journals. J Bone Joint Surg Am. 2005; 87:2632–8.

10. Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003; 85:1–3.

11. Wright JG, Einhorn TA, Heckman JD. Grades of recom-mendation. J Bone Joint Surg Am. 2005; 87:1909–10.

12. Smith GC, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003; 327:1459–61.

13. Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000; 342:1878–86.

14. Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000; 342:1887–92.

15. Carr AJ. Evidence-based orthopaedic surgery: what type of research will best improve clinical practice? J Bone Joint Surg Br. 2005; 87:1593–4.
16. Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980; 66:271–3.
17. Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive mea-sure of disability in low-back pain. Spine (Phila Pa 1976). 1983; 8:141–4.
18. Deyo RA, Diehl AK. Measuring physical and psychosocial function in patients with low-back pain. Spine (Phila Pa 1976). 1983; 8:635–42.

19. Bombardier C. Outcome assessments in the evaluation of treatment of spinal disorders: summary and general recommendations. Spine (Phila Pa 1976). 2000; 25:3100–3.
Figures and Tables%
Fig. 2.
Diagram showing basic design of case-control study. Study groups are determined by outcomes: Patients with a particular outcome are cases, whereas patients without the outcome are controls. This study design looks retrospectively to determine if there is a difference in rate of exposure to a particular variable between cases and controls.
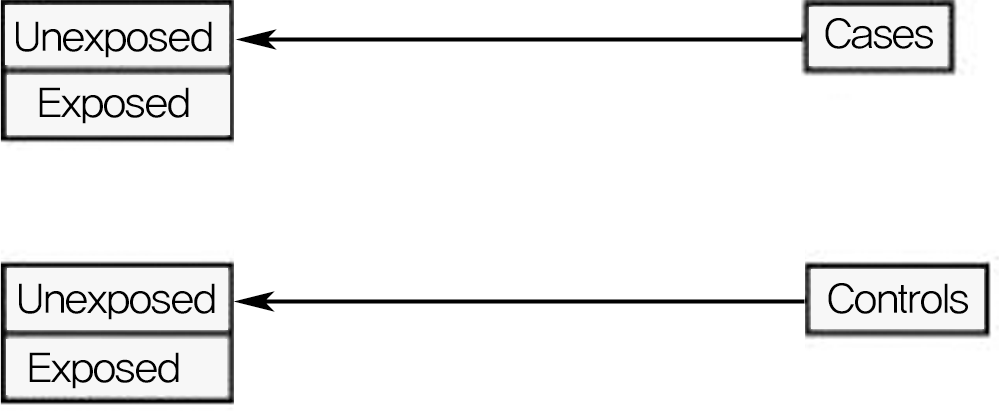
Fig. 3.
Diagram showing basic design of observational cohort study. Treatment is chosen by patient and physician rather than through randomization. Study groups are defined by treatment, and outcomes are compared. Cohort studies can be prospective or retrospective
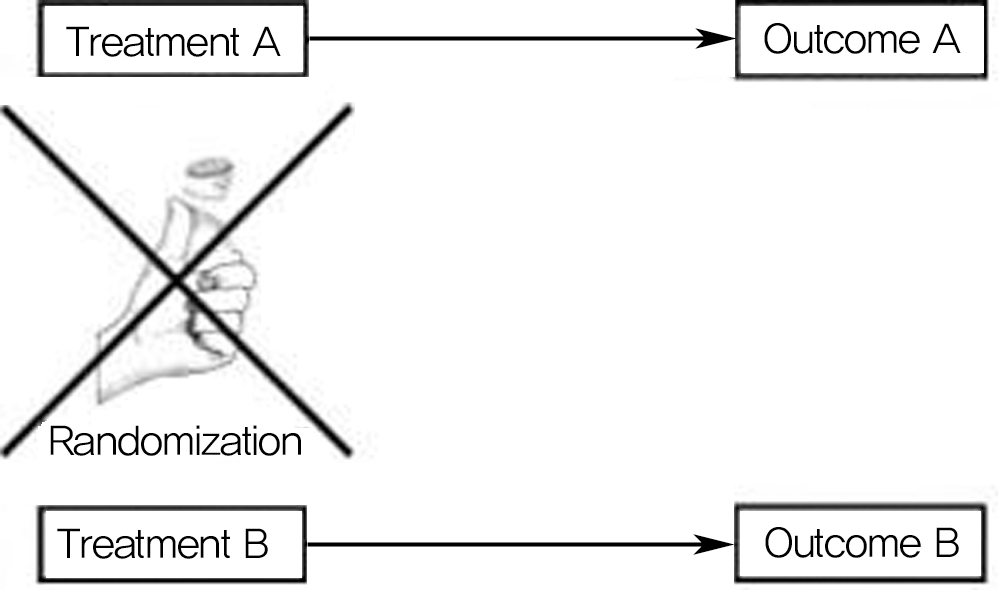
Fig. 4.
Diagram showing basic design of randomized controlled trial. Study sample is randomized to different treatments, and outcomes are prospectively determined
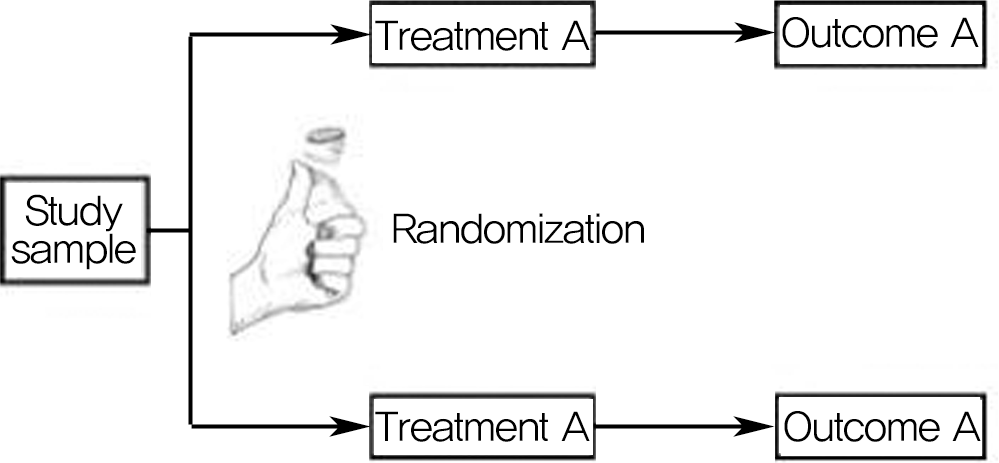
Table 1
* All patients were enrolled at the same point in their disease with ≥80% followup of enrolled patients.† A study of results from two or more previous studies.‡ Patients were compared with a control group of patients treated at the same time and institution § The study was started after treatment was performed.# Patients with a particular outcome (“cases” with, for example, a failed arthroplasty were compared with those who did not have the outcome (‘‘controls’'with, for example, a total hip arthroplasty that did not fail)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download