Abstract
Molecular interactions between epithelium and mesenchyme are important for root formation. Nuclear factor I-C (Nfic) has been identified as a key regulator of root formation. However, the mechanisms of root formation and their interactions between Hertwig's epithelial root sheath (HERS) and mesenchyme remain unclear. In this study, we investigated the role of Nfic in root patterning and growth during molar root development. The molars of Nfic knockout mice exhibited an enlarged pulp chamber and apical displacement of the pulpal floor, characteristic features of taurodontism, due to delayed furcation formation. In developing molar roots of mutant mice at P14, BrdU positive cells decreased in the apical mesenchyme of the elongation region whereas those cells increased in the dental papilla of the furcation region. Whereas cytokeratin 14 and laminin were localized in HERS cells of mutant molars, Smoothened (Smo) and Gli1 were downregulated in preodontoblasts. In contrast, cytokeratin 14 and Smo were localized in the cells of the furcation region of mutant molars. These results indicate that Nfic regulates cell proliferation in the dental mesenchyme and affects the fate of HERS cells in a site-specific manner. From the results, it is suggested that Nfic is required for root patterning and growth during root morphogenesis.
Interactions between dental epithelium and mesenchyme are important for root formation similar to crown formation. While the molecular mechanisms of early tooth development and crown morphogenesis have been extensively studied, little is known about the molecular mechanisms controlling tooth root formation [12].
To date, several signaling pathways have been implicated in tooth root development [345]. Epithelial sonic hedgehog (Shh) and transforming growth factor-β signaling are essential for Hertwig's epithelial root sheath (HERS) formation [46]. In contrast, several signaling molecules are known to be necessary for root elongation in the dental mesenchyme. Ptc1 and Smad4 have been reported to be associated with root elongation. Ptc1 and Smad4 are known to be necessary for root elongation in the dental mesenchyme. Ablation of these genes in the dental mesenchyme results in molars with short roots [37]. Furthermore, conditional disruption of β-catenin in the dental mesenchyme leads to complete absence of roots with HERS formation [5]. These reports strongly suggest that mesenchymal cell proliferation and differentiation may play important roles in root elongation whereas epithelial proliferation is essential for HERS formation during initiation of root formation.
Nuclear factor I-C (Nfic), a DNA-binding transcription factor, has been identified as a key regulator of root formation [89]. Nfic knockout mice exhibit short molar roots [8]. This remarkable tooth phenotype is caused by impaired odontogenic cell proliferation and odontoblast differentiation [10]. Furthermore, it has been recently reported that epithelial Smad4 signaling, essential for HERS formation, is mediated by the Smad4-Shh-Nfic signaling cascade [4]. Interaction between HERS and dental mesenchyme may guide the size, shape, and number of tooth roots. On the basis of previous reports, it is postulated that Nfic may participate in root morphogenesis through interactions between HERS and dental mesenchyme.
Currently, developmental mechanisms underlying root morphogenesis are poorly understood. Morphologically, root morphogenesis is initiated with HERS formation after crown morphogenesis. With the elongation of HERS, mesenchymal cells in the dental papilla proliferate and differentiate into odontoblasts [11]. Recently, it has been reported that mesenchymal proliferation contributes to root morphogenesis [12]. Spatially regulated mesenchymal proliferation is required for creating cylindrical root structure. Mesenchyme in the root elongation region shows relatively higher proliferation than the furcation region. However, the mechanisms of root morphogenesis and their interactions between HERS and dental mesenchyme remain unclear. In addition, it is not known whether Nfic is involved in root morphogenesis. Here, we analyzed Nfic knockout mice to investigate the role of Nfic in root patterning and growth during molar root development.
All experimental procedures were approved by the Animal Welfare Committee of the Chonbuk National University. Nfic null mice have been previously described [89]. Total of 72 animals were used in this study. For histological analysis, mice were sacrificed and the mandibles were carefully dissected. The tissues were fixed in 4% paraformaldehyde (PFA) and decalcified in 10% ethylenediaminetetraacetic acid solutions for 2 to 4 weeks at 4℃. The decalcified tissues were dehydrated through a graded ethanol series, embedded in paraffin, and sectioned with 5-µm thickness. Slides were stained with hematoxylin and eosin.
For immunostaining, sections were treated with 3% hydrogen peroxide, and incubated with rabbit polyclonal antibodies against Nfic (1:200) [13], cytokeratin 14 (CK14; 1:900, Covance, Berkeley, CA, USA), Smo (1:100, Abcam, Cambridge, MA, USA), Gli1 (1:400, LifeSpan Biosciences, Inc., Seattle, WA, USA), laminin (1:100, Abcam), and Phex (1:50, Sigma-Aldrich, St. Louis, MO, USA). A Histostain Plus Rabbit Primary (DAB) kit (Zymed Laboratories, San Francisco, CA, USA) was used according to the manufacturer's instructions.
To detect cell proliferation in developing roots, 5'-bromo-2' deoxyuridine (BrdU) labeling reagent (45 µg/g body weight; Roche, Indianapolis, IN, USA) was injected intraperitoneally into 10-day-old wild type (WT) and mutant (MT) mice. Two hours after injection, mice were dissected, the mandibles were fixed with Carnoy's fixatives at 4℃ overnight, embedded in paraffin, and sectioned in the root elongation region and furcation region for immunodetection with the BrdU labeling and detection kit (Roche). For statistical analysis, 5 consecutive sections from 3 independent littermates were used.
To isolate molars, skin and muscles were clipped away from the dissected mandibles of 14-, 18-, and 28-day-old (P28) mice. The mandibles were then incubated in 50 mM Tris-Cl (pH 8.0), 0.5% sodium dodecyl sulfate, and 0.2 mg proteinase K/ml at 55℃ for 1 hour. After a brief wash in water, the first molars were extracted, fixed in 4% PFA, and immersed in a 5% sodium hypochlorite solution for 1 hour to remove soft tissues. The specimen surfaces were sputter-coated with platinum after drying and examined with a scanning electron microscope (JSM-6400, JEOL, Tokyo, Japan) under 20-kV conditions.
All data are presented as mean±standard error of the mean. All statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical differences were determined by the Student's t test and values of P<0.05 were considered statistically significant.
To observe the morphological features of molar roots during development, we isolated the mandibular first molars of mice at P14, P18, and P28. Under observation with scanning electron microscopy, roots were gradually elongated with age in WT mice but roots were short due to disrupted elongation in MT mice (Fig. 1A-G). In examination of the basal aspect of molar roots, root furcation between mesial and distal roots was regularly observed in WT mice (Fig. 1H, J, L). However, furcation was not observed in MT mice at P14 and P18. In MT mice at P28, apically displaced incomplete furcation was observed (Fig. 1G, N). No furcation was occasionally observed in MT mice (25% penetrance; n=8).
To compare the histological differences between the root elongation and furcation region during root development, we observed hematoxylin and eosin stained frontal sections across the center of the mesial root and mid-furcation of mandibular first molars (Fig. 2A-H). At P8, there was no remarkable difference in the elongation region between WT and MT mice (Fig. 2A, B). In the furcation region of WT mice, thin dentin was observed, but elongated HERS without dentin was observed in MT mice (Fig. 2E, F). At P28, root length was extremely short in the elongation region of MT mice (Fig. 2C, D). In the furcation region of WT mice, relatively thick inter-radicular dentin dentin at the furcation region was formed close to the cervix, but inter-radicular dentin it was very thin and apically displaced in MT mice (Fig. 2G, H).
Because histological differences between WT and MT mice were apparent in the mandibular first molar at P10, we performed BrdU-labeling experiments to assess cell proliferation changes following disruption of Nfic. In WT mice, BrdU-labeled cells were mainly located around HERS in the elongation region, but scarcely found in the pulp core and the furcation region (Fig. 2I, K). In the elongation region, BrdU-labeled cells were significantly reduced in MT compared with WT littermates (Fig. 2J, M). However, BrdU-labeled cells were increased in the furcation region of MT (Fig. 2L, M).
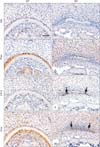
We performed immunohistochemical stains to assess molecular changes in the developing molar roots following disruption of Nfic. Comparable molecular changes in the developing molar roots following Nfic disruption were observed at P10 (Fig. 3). In the elongation region, HERS cells of both WT and MT mice were marked by CK14 (Fig. 3A, E). HERS was thin and short in WT mice, but was longer in MT mice. Laminin, a component of the basement membrane, was localized around HERS both of WT and MT mice (Fig. 3B, F). Localization of Smo, a signal transducer of Shh signaling [6], was overlapped in the membrane of HERS cells in WT mice (Fig. 3C). However, Smo was downregulated in the HERS cells of MT mice. Weak Smo immunoreactivities were observed in the region between the inner layer of HERS and adjacent pulp cells (Fig. 3G). Gli1, a transcription factor activated by Shh [14], was widely localized in odontoblasts, pulp cells, and HERS cells of WT mice (Fig. 3D). In MT mice, Gli1 was also widely localized in the cells of HERS and pulp core except preodontoblasts, dental papilla cells close to the inner layer of HERS (Fig. 3H). In the furcation region of WT mice at P14, Nfic was localized in the odontoblasts and cells in the pulp and periodontium, whereas no immunoreactivity was observed in MT mice (Fig. 4A, B). Phex, a marker for mature odontoblasts, was localized in the odontoblasts of both WT and MT mice (Fig. 4C, D). Both in WT and MT mice, the epithelial rests below the inter-radicular dentin dentin at the furcation region were marked by CK14 (Fig. 4E, F). In WT mice, Smo was localized in the odontoblasts and cells in the periodontium (Fig. 4G). Localization of Smo was found around the epithelial cells in MT mice (Fig. 4H).
Root morphogenesis is controlled by interactions between HERS and dental mesenchyme. Nfic has been identified as a key regulator of root formation. A previous report showed that Nfic knockout mice exhibit short molar roots [8]. In this study, we investigated the roles of Nfic in root morphogenesis. Nfic knockout mice exhibited remarkable tooth phenotypes characterized by an enlarged pulp chamber and apical displacement of the pulpal floor, characteristic features of taurodontism (OMIM 272700) [15]. This abnormality was closely associated with spatial impairment of cell proliferation in the dental mesenchyme.
In the developing roots of Nfic knockout mice, cell proliferation in the apical mesenchyme decreased in the root elongation region but remarkably increased in the root furcation region. This result indicates that mesenchymal cell proliferation was altered depending on the location following disruption of Nfic. Such a spatial regulation of cell proliferation may contribute to abnormal root morphogenesis in Nfic knockout mice. Interestingly, Sohn et al. [12] recently reported that mesenchymal proliferation contributes to root morphogenesis. Mesenchyme in the root elongation region showed relatively higher proliferation than the furcation region. Taken together, it is suggested that short molar roots with impaired furcation formation may be caused by altered cell proliferation in Nfic knockout mice. Moreover, spatial regulation of mesenchymal cell proliferation may be required for root morphogenesis.
It is generally accepted that HERS is important for tooth root formation [16]. HERS is involved in determining root shape and size. However, the molecular mechanisms underlying root morphogenesis remain largely unknown. Previously, it has been reported that HERS fails to form in mice with K14-Cre mediated ablation of Smo or Smad4 in the dental epithelium [46]. In addition, it was also reported that epithelial Smad4 signaling is mediated by the Smad4-Shh-Nfic signaling cascade during HERS formation [4]. These reports indicate that epithelial-mesenchymal interactions mediated by the Smad4-Shh-Nfic signaling cascade-mediated are crucial for initiation of root formation. However, it is questionable whether this signaling cascade remains effective during root elongation and patterning. HERS structures have been observed in disrupted molar roots of several gene targeted mice [357]. In these mutant molars, HERS initially forms but is disrupted during root elongation accompanied with impaired cell proliferation in the dental mesenchyme. In Nfic knockout mice, HERS was formed and elongated downward for a while, but was degenerated with impaired cell proliferation of mesenchyme. These results indicate that mesenchymal cell proliferation may be crucial for determining the fate of HERS for root elongation. Our findings, together with previous evidence, suggest that mesenchymal cell proliferation and differentiation may play important roles in root elongation whereas epithelial proliferation is essential for HERS formation during initiation of root formation.
In the developing roots of Nfic knockout mice, Smo expression was observed in HERS of both the elongation and furcation region at early root formation (P8). At late root formation (P14), Smo was expressed around HERS of the furcation region but downregulated from the elongation region. Furthermore, Gli1 was disappeared in the preodontoblasts of elongation region. These results indicate that ablation of Nfic caused disruption of Shh signaling in preodontoblasts through the downregulation of Smo expression during the late root formation stage. These results suggest that failure of HERS extension in the elongation region of Nfic knockout mice molars may result from a lack of preodontoblast proliferation due to loss of responsiveness to Shh signaling, and that prolonged HERS extension in furcation region may result from increase of cell proliferation. Therefore, Nfic may be involved in determining the fate of HERS through the temporospatial regulation of mesenchymal cell proliferation during root elongation. In this study, we could not determine which molecular signaling spatially regulates responsiveness to Shh signaling in preodontoblasts and HERS following the alteration of mesenchymal cell proliferation. It awaits further investigation to understand the complete mechanisms of root elongation and patterning.
In conclusion, our data suggest that Nfic plays important roles in root patterning and growth through the regulation of mesenchymal cell proliferation. Furthermore, our data provide new insights into understanding the developmental mechanisms of root formation and the pathological mechanism of developmental root anomalies.
Figures and Tables
Fig. 1
Scanning electron microscopy of impaired root patterning in the developing mandibular first molars of nuclear factor I-C (Nfic) knockout mice. (A-G) Lateral/baso-lateral view of molar roots. Roots were gradually elongated with age in wild type (WT) mice, whereas root elongation was impaired in mutant (MT) mice. (H-N) Basal view of molar roots. In WT mice, root furcation between mesial and distal roots was regularly observed. In contrast, furcation was not observed in MT mice. At 28 days old (P28), apical displacement of furcation was observed, but no furcation was occasionally observed (25% penetrance; n=8). The white arrows indicate furcation. Scale bars=200 µm.
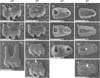
Fig. 2
Histology and cell proliferation analysis in the mandibular first molar of nuclear factor I-C (Nfic) knockout mice. (A-D) Comparison of elongation region in wild type (WT) and mutant (MT) mice at 8 days old (P8) and P28. (E-H) Furcation region. (I-L) BrdU labeling of proliferating cells in the apical dental mesenchyme of developing mandibular molar roots in Nfic knockout mice at P10. (M) Differential regulation of cell proliferation between the elongation and furcation region. In MT mice, cell proliferation was reduced in the elongation region but increased significantly in the furcation region. Scale bars=400 µm (A-H), 100 µm (I-L).
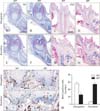
Fig. 3
Molecular changes in the root elongation region of developing mandibular first molars of nuclear factor I-C (Nfic) knockout mice at 10 days old (P10). (A-D) In wild type (WT) mice, cytokeratin 14 (CK14) was localized limitedly in Hertwig's epithelial root sheath (HERS) cells. Laminin and Smo were localized in cell membrane of HERS. In contrast, Gli1 was abundantly localized in the odontoblasts, pulp cells, and HERS cells. (E-H) In mutant (MT) mice, CK14 was diffusely localized in HERS cells. Laminin and Smo were downregulated in the inner layer of HERS. Localization of Gli1 was disappeared in dental papilla cells near the inner layer of HERS. The black arrows indicate HERS and the asterisks indicate cells devoid of Gli1 expression. Ab, alveolar bone; Od, odontoblasts; P, pulp. Scale bar=80 µm.

Fig. 4
Localization of nuclear factor I-C (Nfic), Phex, cytokeratin 14 (CK14), and Smo in the furcation region of developing mandibular first molar of Nfic knockout mice at P14. (A, B) In wild type (WT) mice, Nfic was localized in odontoblasts and cells in the pulp and periodontium, whereas no immunoreactivity was observed in mutant (MT) mice. (C, D) Phex was localized in odontoblasts of both WT and MT mice. (E, F) CK14 was localized in the epithelial rests below the inter-radicular dentin dentin at the furcation region in both WT and MT mice. (G, H) Smo was localized in the odontoblasts and cells in the periodontium in WT mice. In MT mice, localization of Smo was found around Hertwig's epithelial root sheath (HERS). The black arrows indicate HERS. Ab, alveolar bone; P, pulp; Ird, inter-radicular dentin dentin at the furcation region; Od, odontoblasts. Scale bar=80 µm.
Acknowledgements
We thank Dr. Richard Gronostajski for kindly providing Nfic knockout mice. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2013R1A2A1A01007642).
References
1. Tummers M, Thesleff I. The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol. 2009; 312B:309–319.
2. Foster BL, Nociti FH Jr, Somerman MJ. Tooth root development. In : Huang GT, Thesleff I, editors. Stem Cells in Craniofacial Development and Regeneration. Hoboken: Wiley-Blackwell;2013. p. 153–177.
3. Nakatomi M, Morita I, Eto K, Ota MS. Sonic hedgehog signaling is important in tooth root development. J Dent Res. 2006; 85:427–431.
4. Huang X, Xu X, Bringas P Jr, Hung YP, Chai Y. Smad4-Shh-Nfic signaling cascade-mediated epithelial-mesenchymal interaction is crucial in regulating tooth root development. J Bone Miner Res. 2010; 25:1167–1178.
5. Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, Cho ES. Beta-catenin is required in odontoblasts for tooth root formation. J Dent Res. 2013; 92:215–221.
6. Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development. 2002; 129:5323–5337.
7. Gao Y, Yang G, Weng T, Du J, Wang X, Zhou J, Wang S, Yang X. Disruption of Smad4 in odontoblasts causes multiple keratocystic odontogenic tumors and tooth malformation in mice. Mol Cell Biol. 2009; 29:5941–5951.
8. Steele-Perkins G, Butz KG, Lyons GE, Zeichner-David M, Kim HJ, Cho MI, Gronostajski RM. Essential role for NFI-C/CTF transcription-replication factor in tooth root development. Mol Cell Biol. 2003; 23:1075–1084.
9. Park JC, Herr Y, Kim HJ, Gronostajski RM, Cho MI. Nfic gene disruption inhibits differentiation of odontoblasts responsible for root formation and results in formation of short and abnormal roots in mice. J Periodontol. 2007; 78:1795–1802.
10. Lee DS, Park JT, Kim HM, Ko JS, Son HH, Gronostajski RM, Cho MI, Choung PH, Park JC. Nuclear factor I-C is essential for odontogenic cell proliferation and odontoblast differentiation during tooth root development. J Biol Chem. 2009; 284:17293–17303.
11. Thomas HF. Root formation. Int J Dev Biol. 1995; 39:231–237.
12. Sohn WJ, Choi MA, Yamamoto H, Lee S, Lee Y, Jung JK, Jin MU, An CH, Jung HS, Suh JY, Shin HI, Kim JY. Contribution of mesenchymal proliferation in tooth root morphogenesis. J Dent Res. 2014; 93:78–83.
13. Bae CH, Kim TH, Chu JY, Cho ES. New population of odontoblasts responsible for tooth root formation. Gene Expr Patterns. 2013; 13:197–202.
14. Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998; 125:2803–2811.
15. Wright T. The molecular control of and clinical variations in root formation. Cells Tissues Organs. 2007; 186:86–93.
16. Ten Cate AR. The role of epithelium in the development, structure and function of the tissues of tooth support. Oral Dis. 1996; 2:55–62.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download