Abstract
The paracolpium or paravaginal tissue is surrounded by the vaginal wall, the pubocervical fascia and the rectovaginal septum (Denonvilliers' fascia). To clarify the configuration of nerves and fasciae in and around the paracolpium, we examined histological sections of 10 elderly cadavers. The paracolpium contained the distal part of the pelvic autonomic nerve plexus and its branches: the cavernous nerve, the nerves to the urethra and the nerves to the internal anal sphincter (NIAS). The NIAS ran postero-inferiorly along the superior fascia of the levator ani muscle to reach the longitudinal muscle layer of the rectum. In two nulliparous and one multiparous women, the pubocervical fascia and the rectovaginal septum were distinct and connected with the superior fascia of the levator at the tendinous arch of the pelvic fasciae. In these three cadavers, the pelvic plexus and its distal branches were distributed almost evenly in the paracolpium and sandwiched by the pubocervical and Denonvilliers' fasciae. By contrast, in five multiparous women, these nerves were divided into the anterosuperior group (bladder detrusor nerves) and the postero-inferior group (NIAS, cavernous and urethral nerves) by the well-developed venous plexus in combination with the fragmented or unclear fasciae. Although the small number of specimens was a major limitation of this study, we hypothesized that, in combination with destruction of the basic fascial architecture due to vaginal delivery and aging, the pelvic plexus is likely to change from a sheet-like configuration to several bundles.
The rectovaginal septum covers the posterior aspect of the vagina, whereas the pubocervical fascia covers the anterior aspect; both fasciae provide so-called level II support to the female pelvic viscera, preventing prolapse [1]. Mostwin [2] considered the pubocervical fascia to be a tissue filling the space between the vagina and the urethra or bladder. The tendinous arch of the pelvic fascia (arcus tendineus fasciae pelvis) has been a common target in studies of gross anatomy [3, 4, 5]. Along the tendinous arch, the rectovaginal septum and pubocervical fascia meet the superior fascia of the levator ani, the two structures being apparently embedded in the lower paracolpium or the paravaginal tissue [1]. The rectovaginal septum corresponds to the Denonvilliers' fascia in women [6]. Because the mesorectum (a fatty tissue layer surrounding the lower rectum) is adjacent to the Denonvilliers' fascia in women [7], the covering fascia of the mesorectum most likely joins, or correspond to, the rectovaginal septum. These features of the fasciae along and around the vagina are summarized in Fig. 1.
Donker [8] appers to have been the first researcher to have noted abundant thick nerves running through the paracolpium from the pelvic plexus to the urologic organs, and his observations were followed up by Ball et al. [9]. Thus, the paracolpium is likely to include at least some of the pelvic autonomic nerve plexus (Fig. 1). Using late-stage fetuses, Kato et al. [10] demonstrated the female cavernous nerve running through the putative paracolpium. Hirata et al. [11] provided an elegant diagram showing the course of the female cavernous nerve covering the pubocervical fascia, but they did not provide a photographic figure of the nerve. To identify the cavernous nerve, immunohistochemistry for neuronal nitric oxide synthase (nNOS) is necessary [12]. Using sections from a 21-week-old fetus, Alsaid et al. [13] demonstrated and reconstructed the female cavernous nerve running alongside the rectum and vagina. However, reports describing the application of nNOS immunohistochemistry to the adult pelvic nerves seem to be very limited, possibly because of difficulty in obtaining good materials for the immunoreaction [14]. Likewise, the application of immunohistochemistry for vasoactive intestinal peptide (VIP) in adult tissues seems to have been limited to a study of intramural nerves in the vagina [15].
Any dissection study designed to observe the topographical anatomy including nerves and fasciae in the pelvis may be limited because of the delicate architecture: minute dissection of autonomic nerves [16, 17] may not be appropriate for demonstration of pelvic fasciae, as was shown in two elegant studies of fresh cadavers published in the same year [18, 19]. There have been several attempts to show both the nerves and the fascia: using frozen cadavers, Hollabaugh et al. [20] demonstrated an "intrapelvic" course of the female pelvic splanchnic nerves alongside the extrapelvic pudendal nerve as a result of broken fasciae. Likewise, Mostwin [2] demonstrated these nerves through windows artificially created in the fasciae. Conversely, histological descriptions of female topographical anatomy among the fasciae and intrapelvic nerves along the tendineous arch require large sections that include related structures, and this seems to have limited the number of such studies [6, 21]. Although large sections are good for demonstration of the pelvic fasciae, but they are not suitable for immunohistochemistry because of their thickness and/or acidic decalcification. Therefore, using classical paraffin sections, the aim of the present study was to demonstrate in detail the topographical anatomy of both the nerves and fasciae in the paracolpium with the aid of immunohistochemistry.
This study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined 10 donated female cadavers ranging in age from 73 to 100 years, with a mean age of 85 years. The cadavers included two nulliparous women (Table 1). In all cases the cause of death had been ischemic heart failure or intracranial bleeding and we confirmed that none of the women had undergone surgery by reference to their individual histories as well as macroscopic observation after opening the abdominopelvic cavity. These cadavers had been donated to Sapporo Medical University for research and education on human anatomy, and their use for research had been approved by the university ethics committee. The donated cadavers had been fixed by arterial perfusion of 10% v/v formalin solution and stored in 50% v/v ethanol solution for more than 3 months. From each cadaver, we prepared one large tissue block including the bladder, urethra, uterus, vagina, rectum, levator ani muscle, and any connective tissue around these viscera. Five to seven slices (mostly 15 mm in thickness) were prepared from each of the hemiblocks after bisection along the midsagittal line, and after performing routine procedures for paraffin-embedded histology, five to seven large sections (70×50 mm) stained with hematoxylin and eosin (H&E) were prepared at 2-3-mm intervals from each of the slices. The sectional planes corresponded to tilted frontal planes according to Tamakawa et al. [21] in order to show the bladder, uterus (or vagina), and rectum together in a single plane. After observation of the large sections, we prepared normal-size sections for immunohistochemistry near the former large section. Thus, from one paraffin block containing a 15-mm-thick slice, we prepared 3-4 large sections and 15-20 normal-size sections.
Most sections were stained with H&E and some were used for elastica Masson staining for elastic fibers [22, 23] and immunohistochemistry. The primary antibodies used for nerve immunohistochemistry were 1) mouse monoclonal anti-human S100 protein (1:100, Dako Z0311, Dako, Glostrup, Denmark), 2) rabbit polyclonal anti-human neuronal nitric oxide synthase or nNOS (1:100, Cell Signaling Technology, Beverly, MA, USA), 3) mouse monoclonal anti-superolateral part, while nNOS-andhuman vasoactive intestinal peptide (H-6) or VIP (1:100, sc25347, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and, 4) rabbit polyclonal anti-human tyrosine hydroxylase (TH; 1:100, ab152, Millipore-Chemicon, Temecula, CA, USA). Rather than PGP9.5, neuron-specific enolase and PMP22, we have found that S100 protein is the best marker in long-preserved cadaveric specimens [10, 24, 25, 26]. In addition to elastica Masson staining, to understand the connective tissue architecture, we used mouse monoclonal anti-human alpha smooth muscle actin (1:100, Dako M0851, Dako) as the primary antibody. For all primary antibodies, the secondary antibody was labeled with horseradish peroxidase (HRP), and antigen-antibody reactions were detected by the HRP-catalyzed reaction with diaminobenzidine. Counterstaining with hematoxylin was performed on the same samples. A negative control without a first antibody was set up for each of the specimens. Observations and photography were usually performed with a Nikon Eclipse 80 (Nikon, Tokyo, Japan), but photos at ultralow magnification (less than ×1 at the objective lens) were taken using a high-grade flat scanner with translucent illumination (Epson scanner GTX970, Tokyo, Japan).
The present large sections covered a wide area including the anal sphincter at the posterior end, the urethra at the anterior end, the ureter at the superior end, and the levator ani muscle and ischiorectal fossa along the lateral margin. The retrorectal course of the hypogastric nerve as well as the proximal course of the pelvic splanchnic nerve lay outside the sections. Lower-magnification histology, shown in Figs. 2, 3, 4, 5, 6, corresponded to areas almost 2/3 of the sectional area. Figs. 2 and 3 (or 4, 5, 6) demonstrate the morphology of nuliiparous (or multiparous) women. For convenience, macroscopic slices before histological procedures are shown at the top of Figs. 2 and 4, although the histological sections display planes deeper than the surface of the slice. Using elastica-Masson staining as well as immunohistochemistry for alpha smooth muscle actin, components of the connective tissue fibers are shown at the bottom of the same figures. Likewise, for nerve fibers in the paracolpium (Figs. 3, 5, 6), immunoreactivity for nNOS and VIP was used for indicating parasympathetic nerves and that for TH was used for indicating sympathetic nerves. We used Auerbach's plexus of the rectum for monitoring any false-negative reaction for nNOS or VIP (figures not shown). As a result, nNOS (or VIP) reactivity in the rectum was identified in 7 (or 8) of the 10 cadavers, but not in the other 3, possibly due to poor preservation after death (Table 1). In contrast, reactivity for S100 or TH was consistently observed.
The rectovaginal septum (Denonvilliers' fascia) was consistently seen as a fibrous boundary facing the mesorectal loose tissue in the medial area near the midsagittal line, but it was fragmented or unclear in the lateral area (i.e., the paracolpium) in 5 specimens (multiparous or uniparous women) (Fig. 4E). The rectovaginal septum was composed of a mixture of collagenous and elastic fibers (Fig. 4I). The pubocervical fascia was consistently unclear in the medial area, but identifiable as a rough fibrous network of thick fiber bundles in the lateral area. The latter fascia provided a boundary for the paracolpium facing the thick subperitoneal fatty tissue (Fig. 2D-F). The pubocervical fascia and the rectovaginal septum did not contain smooth muscles (Fig. 2G, H). In contrast, the superior fascia of the levator contained or accompanied smooth muscles at sites near the rectum (Fig. 2I), the smooth muscles being continuous with the adjoining longitudinal muscle coat of the anal canal.
Notably, in 3 of the 10 cadavers examined, we found definite fascial connections at the tendineous arch of the pelvic fasciae; therein, the rectovaginal septum and the pubocervical fascia were each independently connected with the superior fascia of the levator ani (nulliparous, 2; multiparous, 1) (Fig. 2E, F). In these 3 cadavers, nerves in the paracolpium were almost evenly distributed to form a sheet-like configuration; at levels below the uterine cervix, the density was higher in the lateral area near the superior fascia of the levator ani than in the medial area (Fig. 3A-C). The nerves were embedded in elastic fiber-rich tissues. In a single cadaver (uniparous), any clear fascial connection was restricted to the upper part of the vagina. In another cadaver (multiparous) (Fig. 6A), the rectovaginal septum was connected with the superior fascia of the levator ani, but the pubocervical fascia appeared to be replaced by the venous plexus. The venous plexus was embedded in elastic fiber-rich, loose tissues of the paracolpium (Fig. 4H). In the other 5 cadavers, the fascial connection at the tendinous arch appeared to be replaced by the venous plexus (Fig. 4D-F).
Thicker nerves tended to be located in the lateral part of the paracolpium, whereas thinner nerves were located in the medial area (Figs. 3, 5). Abundant nerves in the posterosuperior area of the sections (i.e., the space between the distal ureter and the uterine cervix) (Figs. 2A, 4A) appeared to correspond to the so-called detrusor nerves extending toward a site in the bladder at and around the opening of the ureter [27]. In the paracolpium, most of the nerves were cut transversely in the present sections, and thus appeared to run mostly along the postero-anterior axis. Because 2-5 ganglion cell clusters were seen in the paracopium in each of the sections (Figs. 3, 5), the paracolpium appeared to contain the distal or peripheral part of the pelvic autonomic nerve plexus and its branches such as the cavernous nerve, the nerves to the urethra, and the nerves to the internal anal sphincter (NIAS). The plexus and its distal branches were sandwiched by the pubocervical fascia and the rectovaginal septum. The paravaginal ganglion cells and nerves were composed of a mixture of sympathetic and parasympathetic nerve elements (Fig. 5D-F), but the ratio varied significantly between sites: TH-positive sympathetic nerve ganglion cells tended to be dominant in the superolateral part, while nNOS- and/or VIP-positive parasympathetic cells were distributed widely. Most of the nNOS-positive ganglion cells were also positive for VIP (i.e., double positive) (Fig. 3D, E). The NIAS ran postero-inferiorly along the superior fascia of the levator to reach the longitudinal muscle layer of the rectum (Figs. 3A, 5C). The longitudinal course of the cavernous and urethral nerves was seen to originate from the paracolpium and run alongside the urethra in 2 specimens (both, multiparous) (Fig. 6). In the 5 specimens with a thick venous plexus, nerves in the paracolpium were divided into the superior and inferior clusters (Fig. 5C); the superior group appeared to contain candidates for the cavernous and urethral nerves, while the inferior group issued the NIAS. Conversely, few thin nerves were evident between veins. Finally, in the relatively inferior or distal planes we demonstrated, branches (tributaries) of the superior rectal artery (vein) were accompanied by a few thin nerves in the mesorectum behind the rectovaginal septum.
The rectovaginal septum (Denonvilliers' fascia) as well as the pubocervical fascia is a well-known gynecologic landmark. The rectovaginal septum has been a target of histological studies [28, 29]. However, demonstration of both fasciae in a single histological section has been rare: Tamakawa et al. [21] did so, but they did not use the common terminology. Hirata et al. [11, 30] noted both of the fasciae, but these were beyond the scope of their study. Our present observations of nulliparous women showed that each of the fasciae was independently connected with the superior fascia of the levator ani. A number of researchers have considered the superior fascia of the levator ani to be the so-called "endopelvic fascia" (reviewed by Hirata et al. [30]), but in the present study we did not use this term because there are many different opinions regarding terminology. Although the small number of specimens we examined was a major limitation of the present study, nulliparous women are likely to exhibit the ideal fascial connection at the tendineous arch of the pelvic fasciae, as proposed by DeLancey [1] (Fig. 1). Destruction of the fascial connection is not always evident even in multiparous women, as Hirata et al. [11] have demonstrated. Thus, the tendinous arch seemed to be more resistant to vaginal delivery than the uterosacral ligament, which is histologically fragmented in multiparous women [11]. Moreover, in multiparous women, the fascial connection seemed to be replaced by the venous plexus, and the veins were embedded in elastic fiber-rich, loose paravaginal tissues. There fore, we hypothesized that, due to vaginal delivery and aging, the pelvic plexus is likely to change from a sheet-like configuration to several bundles in combination with destruction of the basic fascial configuration.
Ball et al. [9] stated that the paravaginal nerves are not localized, but "unfortunately" distributed evenly, unlike the male neurovascular bundles at the 5 and 7 o'clock positions in the prostate. This description is consistent with our observations in nulliparous women. Fig. 7A displays a sheet-like configuration comprising almost evenly distributed paravaginal nerves between the fasciae. In multiparous women with a well developed venous plexus in the paracolpium, veins separated the nerves into superior and inferior groups, and thus the paravaginal nerves tended to be bundled into several fasciculi, although Fig. 7B does not demonstrate this feature well because of technical imperfection. Irrespective of whether they were evenly distributed or bundled, the paravaginal nerves contained both sympathetic and parasympathetic elements. Using fetuses, Yucel et al. [12] demonstrated that the proximal course the cavernous course with nNOS reactivity is close to the mid-urethra at the 5 and 7 o'clock positions, and that at the level of the proximal urethra, it joins the paravaginal nerves at the 2 and 10 o'clock positions of the vagina. In the evenly distributed paravaginal nerves, the female cavernous nerve is likely to localize at a specific site. Nevertheless, demonstration of the longitudinal course was difficult, and we were able to do it in only 2 specimens.
The NIAS (nerves supplying the internal anal sphincter) is another important distal branch of the pelvic plexus although it is not widely known than the cavernous nerves. Because the nerves ran along the posterior or inferior side of the paravaginal venous plexus in multiparous women, tension-free vaginal mesh techniques for uterine prolapse are likely to injure it; this status is similar to that of the levator ani nerve [31]. In fact, the levator ani nerve lies very close the NIAS, but the former (or latter) is located laterally (or medially) to the superior fascia of the levator ani muscle (unpublished data). In the relatively inferior or distal planes we demonstrated, branches (tributaries) of the superior rectal artery (vein) did not accompany abundant nerves in the anterior part of the mesorectum. Conversely, in the same planes or those nearby, the paracolpium was located close to the rectal wall, and the superior fascia of the levator ani was rich in smooth muscles. The latter muscles were continuous with the longitudinal muscle coat of the anal canal, as demonstrated by Arakawa et al. [32]. Thus, the so-called lateral ligament of the rectum seemed to connect with the paracolpium or correspond to part of the latter, although it has been suggested that the ligament may be a surgical artifact [33]. The middle rectal vein, i.e., the major and consistent component of the lateral ligament [34], is likely to drain into the paravaginal venous plexus. Recent macroscopic studies of the lateral ligament of the rectum [7, 35, 36] have indicated that it contains nerve bundles. We consider the NIAS to be a "core" nerve of the lateral ligament.
Figures and Tables
Fig. 1
Schematic representation of the pelvic fasciae along and around the vagina. The right-hand side of the figure displays fascial connections with the superior fascia of the levetor anti-muscle at the tendinous arch of the pelvic fasciae: the pubocervical fascia (red line marked A) joins not only the rectovaginal septum (Denonvilliers' fascia; blue line marked B) but also, possibly, a fascia around the mesorectum (yellow line marked C). The mesorectum (a surgical term) does not correspond to the true mesentery but a loose mass of fatty tissue surrounding the lower rectum. The green colored square indicates the plane used for most of photographic demonstrations in the present study: it includes the 3 visceral systems in the pelvis (the urologic system, the reproductive system, and the alimentary canal) as well as the tendinous arch of the pelvic fasciae. The left hand-side of the figure shows a partial dissection of the paravaginal tissue or paracolpium. The paracolpium in the present study is likely to include at least some parts of the pelvic autonomic nerve plexus (pelvic plexus). The bilateral hypogastric nerve (left) is also dissected to show its arrival at the pelvic plexus area. The pelvic splanchnic nerve runs along the anteromedial side of the ischial spine, while the pudendal nerve runs along the posterolateral side. S3, third sacral nerve root.
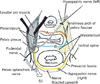
Fig. 2
Histology of a specimen from a 79-year-old nulliparous woman. Tilted frontal planes. Panels (A-C) display macroscopic slices before histologic preparation. Panel (A) represents the most posterosuperior side of the 3 panels, while panel (C) represents the most antero-inferior side. The posterior end of the urinary bladder (B) is seen in panel (C). Stars indicate the superior fascia of the levator ani muscle. Intervals between panels are about 15 mm. Panels (D-F) exhibit hematoxylin and eosin sections at the same lower magnification corresponding to the central parts of panels (A-C), respectively. Scale bar in panel (D)=10 mm (D-F). The rectovaginal septum (Denonvilliers' fas cia; RVS) and the pubocervical fascia (PCF) are seen connecting with the superior fascia (stars) of the levator ani muscle (LA). Panels (G-I; same magnification) correspond to the squares in panels (E) and (F). Scale bar in panel (H, I)=0.1 mm (G-I). Panels (G) and (H) (immunohistochemistry for smooth muscle actin) show smooth muscles in the PCF (G) and recto vaginal septum (H). Panel (I) (elastica-Masson staining) shows smooth muscles and elastic fibers (black lines) in the superior fascia of the levator. ATFP, arcus tendineus fasciae pelvis; EAS, external anal sphincter; IF, ischorectal fossa; OI, obturator internus muscle; R, rectum; SRA&V, branches and tributaries of the su perior rectal artery and vein; UR, ure ter; UT, uterus; VAG, vagina.

Fig. 3
S100 immunohistochemistry of nerves in the paracolpium of the specimen shown in Fig. 2. Panels (A), (B), and (C) (same magnification) cor respond to the central parts of Fig. 2D, E and F, respectively, and display nerves in the paracolpium. Scale bar in panel (A)=1 mm (A-C). S100-positive nerves are distributed almost evenly in the lateral part of the paracolpium. Nerves with arrows contain ganglion cell clusters. Asterisks in panel (A) indicate an artifactual cleft created by the histo logical procedure. Panels (D), (E) and (F) (same magnification) show immunohistochemistry of neuronal nitric oxide synthase (nNOS), vasoactive intestinal peptide (VIP), and tyrosine hydroxylase (TH) in adjacent sections of a ganglion cell cluster in panel (C), respectively. Scale bar in panel (D)=0.1 mm (D-F). Most of the nNOS-positive cells (arrows in panels D and E; parasympathetic) appear to express VIP (i.e., double positive) with a few exceptions (arrowheads). The cluster also con tains TH-positive sympathetic nerve cells (F). Nerves to the internal anal sphincter (NIAS) (or nerves to the levator ani) candidate nerves extending to the internal anal sphincter (those to the levator ani muscle [LA]). PCF, pubocervical fascia; R, rectum; RVS, recto vaginal septum.
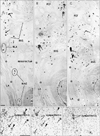
Fig. 4
Histology of a specimen from a 73-year-old multiparous woman. Tilted frontal planes. Panels (A-C) display macroscopic slices before histologic preparation. Panel (A) represents the most posterosuperior side of the 3 panels, while panel (C) represents the most antero-inferior side. The inferior end of the urinary bladder (B) is seen in panel (C). Open stars indicate the superior fascia of the levator ani muscle. Intervals between panels are about 15 mm. Panels (D-F) exhibit hematoxylin and eosin sections at the same lower magnification corresponding to the central parts of panels (A-C), respectively. Scale bar in panel (D)=10 mm (D-F). Parts of the rectovaginal septum (Denonvilliers' fascia; RVS) are seen, but the pubocervical fascia (PCF) is fragmented in panels (A) and (B). Panels (G-J) (same magnification) are higher-magnification views (elastica-Masson staining) of the circles marked G, H, I, and J in panels (E) and (F), respectively. Scale bar in panel (G)=0.1 mm (G-J). Elastic fibers are stained black. A candidate for the perineal membrane (PM) in panel (J) contains regularly arrayed elastic fibers. Black star in panel (D) corresponds to that in Fig. 5A. EAS, external anal sphincter; IF, ischorectal fossa; LA, levator ani muscle; OI, obturator internus muscle; R, rectum; SRA&V, branches and tributaries of the superior rectal artery and vein; UR, ureter; UT, uterus; VAG, vagina.
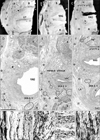
Fig. 5
S100 immunohistochemistry of nerves in the paracolpium of the specimen shown in Fig. 4. Panels (A), (B), and (C) (same magnification) correspond to the central parts of Fig. 4D, E and F, respectively, and display nerves in the paracolpium. Scale bar in panel (A)=1 mm (A-C). Black star in panel (A) corresponds to that in Fig. 4D. In panel (C), the venous plexus divides the nerves into two groups: one of the groups is located close to the superior rectal artery and vein (SRA&V). Nerves with an arrow contain ganglion cell clusters. Panels (D-F) (same magnification) show immunohistochemistry of adjacent sections of a nerve in panel (B). Scale bar in panel (D)=0.1 mm (D-F). The neuronal nitric oxide synthase (nNOS)-positive parasym pathetic nerve fibers (D) as well as tyrosine hydroxylase (TH)-positive sympathetic nerve fibers (F) are evenly distributed in the nerve, while vasoactive intestinal peptide (VIP)-positive parasympathetic nerve fibers tend to be restricted to the right-hand side of the nerve (E). Nerves to the internal anal sphincter (NIAS) (or NLA) candidate nerves extending to the internal anal sphincter (those to the levator ani muscle). Other abbreviations are the same as in Fig. 2. LA, levator ani muscle; VAG, vagina.
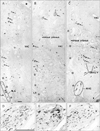
Fig. 6
Candidates for the cavernous and urethral nerves in an 88-year-old multiparous woman. Tilted frontal planes. Panel (A) (hamatoxylin and eosin staining) displays a low-magnification view including the paracolpium. Scale bar in panel (A)=10 mm. Panels (B) and (C) show S100 immunohistochemistry: panel (B) cor responds to the square in the up per margin of panel (C), while panel (C) shows areas along the levator ani muscle (LA) in panel (A). Scale bar in panel (C)=1 mm (B, C). Open star in panels (B) and (C) indicates a same site for comparison. In panel (C), candidates for the cavernous and urethral nerves run anteriorly along the lateral side of the vagina (VAG). Nerves with an arrow contain ganglion cell clusters (B). Panels (D-F) (same magnification) show immunohistochemistry in sections adjacent to the cavernous nerve candidate in panel (C). Scale bar in panel (D)=0.1 mm (D-F). Most of the parasympathetic nerve fibers appear to express both nNOS (D) and VIP (E), i.e., double positive. The nerve also contains tyrosine hydroxylase-positive sympathetic nerve fibers (F). R, rectum; RVS, rectovaginal septum.
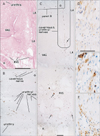
Fig. 7
Hypothetical change in the morphology of the pelvic plexus from a sheet to several bundles in combination with destruction of the covering fascias due to vaginal delivery and aging. Panel (A) displays the basic configuration without destruction of the pelvic fasciae. The pelvic plexus takes a sheet-like configuration comprising a complex of almost evenly distributed nerves. In these branches, the bladder detrusor nerves (DN) were greatest in number, while the cavernous nerve (CN), the nerves to the urethral sphincters (urethral nerve or UN) and the nerves to the internal anal sphincter (NIAS) were relatively fewer. The distal parts of the pelvic plexus and its branches were located in a space between the pubocervical fascia (PCF) and the rectovaginal septum (RVS=Denonvilliers' fascia or DF). Panel (B) shows the change in shape of the plexus in combination with destruction of the definite fasciae along the vagina due to the secondarily developed venous plexus (orange dots). The distal branches are not evenly distributed but form several bundles. ATFL, arcus tendineus fasciae pelvis; B, urinary bladder; HGN, hypogastric nerve; PSN, pelvic splanchnic nerve; R, rectum; UR, ureter; UT, uterus; VAG, vagina.
Table 1
Information of the specimens examined and the tendinous arch morphology

*Neuronal nitric oxide synthase (nNOS) reaction: Auerbach's plexus of the rectum was positive or negative (good or bad) in immunohistochemistry of nitric oxide synthase (nNOS); The pelvic plexus was subdivided into groups or the composite nerves were almost evenly distributed. †Number of chidren with vaginal delivery. ‡Tendinous arch morphology: the attachment site of the pubocervical fascia and/or rectovaginal septum with the superior fascia of the levator ani. §In the lower part of the vagina, the anterolateral corner extended alongside the urethra.
References
1. DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992; 166(6 Pt 1):1717–1724.
2. Mostwin JL. Current concepts of female pelvic anatomy and physiology. Urol Clin North Am. 1991; 18:175–195.
3. Mauroy B, Goullet E, Stefaniak X, Bonnal JL, Amara N. Tendinous arch of the pelvic fascia: application to the technique of paravaginal colposuspension. Surg Radiol Anat. 2000; 22:73–79.
4. Pit MJ, De Ruiter MC, Lycklama AN, Marani E, Zwartendijk J. Anatomy of the arcus tendineus fasciae pelvis in females. Clin Anat. 2003; 16:131–137.
5. Albright TS, Gehrich AP, Davis GD, Sabi FL, Buller JL. Arcus tendineus fascia pelvis: a further understanding. Am J Obstet Gynecol. 2005; 193(3 Pt 1):677–681.
6. Zhai LD, Liu J, Li YS, Yuan W, He L. Denonvilliers' fascia in women and its relationship with the fascia propria of the rectum examined by successive slices of celloidin-embedded pelvic viscera. Dis Colon Rectum. 2009; 52:1564–1571.
7. Zhang C, Ding ZH, Li GX, Yu J, Wang YN, Hu YF. Perirectal fascia and spaces: annular distribution pattern around the mesorectum. Dis Colon Rectum. 2010; 53:1315–1322.
8. Donker PJ. A study of the myelinated fibres in the branches of the pelvic plexus. Neurourol Urodyn. 1986; 5:185–202.
9. Ball TP Jr, Teichman JM, Sharkey FE, Rogenes VJ, Adrian EK Jr. Terminal nerve distribution to the urethra and bladder neck: considerations in the management of stress urinary incontinence. J Urol. 1997; 158(3 Pt 1):827–829.
10. Kato M, Niikura H, Yaegashi N, Murakami G, Tatsumi H, Matsubara A. Histotopography of the female cavernous nerve: a study using donated fetuses and adult cadavers. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:1687–1695.
11. Hirata E, Koyama M, Murakami G, Ohtsuka A, Abe S, Ide Y, Fujiwara H, Kudo Y. Comparative histological study of levels 1-3 supportive tissues using pelvic floor semiserial sections from elderly nulliparous and multiparous women. J Obstet Gynaecol Res. 2011; 37:13–23.
12. Yucel S, De Souza A Jr, Baskin LS. Neuroanatomy of the human female lower urogenital tract. J Urol. 2004; 172:191–195.
13. Alsaid B, Moszkowicz D, Peschaud F, Bessede T, Zaitouna M, Karam I, Droupy S, Benoit G. Autonomic-somatic communications in the human pelvis: computer-assisted anatomic dissection in male and female fetuses. J Anat. 2011; 219:565–573.
14. Hieda K, Cho KH, Arakawa T, Fujimiya M, Murakami G, Matsubara A. Nerves in the intersphincteric space of the human anal canal with special reference to their continuation to the enteric nerve plexus of the rectum. Clin Anat. 2013; 03. 20. [Epub]. http://dx.doi.org/10.1002/ca.22227.
15. Hoyle CH, Stones RW, Robson T, Whitley K, Burnstock G. Innervation of vasculature and microvasculature of the human vagina by NOS and neuropeptide-containing nerves. J Anat. 1996; 188(Pt 3):633–644.
16. Maas CP, DeRuiter MC, Kenter GG, Trimbos JB. The inferior hypogastric plexus in gynecologic surgery. J Gynecol Tech. 1999; 5:55–62.
17. Baader B, Herrmann M. Topography of the pelvic autonomic nervous system and its potential impact on surgical intervention in the pelvis. Clin Anat. 2003; 16:119–130.
18. Ercoli A, Delmas V, Fanfani F, Gadonneix P, Ceccaroni M, Fagotti A, Mancuso S, Scambia G. Terminologia Anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: a dissection-based comparative study. Am J Obstet Gynecol. 2005; 193:1565–1573.
19. Yabuki Y, Sasaki H, Hatakeyama N, Murakami G. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005; 193:7–15.
20. Hollabaugh RS, Steiner MS, Dmochowski RR. Neuroanatomy of the female continence complex: clinical implications. Urology. 2001; 57:382–388.
21. Tamakawa M, Murakami G, Takashima K, Kato T, Hareyama M. Fascial structures and autonomic nerves in the female pelvis: a study using macroscopic slices and their corresponding histology. Anat Sci Int. 2003; 78:228–242.
22. Motohashi O, Suzuki M, Shida N, Umezawa K, Ohtoh T, Sakurai Y, Yoshimoto T. Subarachnoid haemorrhage induced proliferation of leptomeningeal cells and deposition of extracellular matrices in the arachnoid granulations and subarachnoid space. Immunhistochemical study. Acta Neurochir (Wien). 1995; 136:88–91.
23. Hayashi T, Kumasaka T, Mitani K, Yao T, Suda K, Seyama K. Loss of heterozygosity on tuberous sclerosis complex genes in multifocal micronodular pneumocyte hyperplasia. Mod Pathol. 2010; 23:1251–1260.
24. Kato M, Matsubara A, Murakami G, Abe S, Ide Y, Sato I, Usui T. Female perineal membrane: a study using pelvic floor semiserial sections from elderly nulliparous and multiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:1663–1670.
25. Niikura H, Jin ZW, Cho BH, Murakami G, Yaegashi N, Lee JK, Lee NH, Li CA. Human fetal anatomy of the coccygeal attachments of the levator ani muscle. Clin Anat. 2010; 23:566–574.
26. Hinata N, Murakami G, Abe S, Honda M, Isoyama T, Sejima T, Takenaka A. Detailed histological investigation of the female urethra: application to radical cystectomy. J Urol. 2012; 187:451–456.
27. Takenaka A, Soga H, Murakami G, Niikura H, Tatsumi H, Yaegashi N, Tanaka K, Fujisawa M. Understanding anatomy of "hilus" of detrusor nerves to avoid bladder dysfunction after pelvic surgery: demonstration using fetal and adult cadavers . Urology. 2009; 73:251–257.
28. Leffler KS, Thompson JR, Cundiff GW, Buller JL, Burrows LJ, Schön Ybarra MA. Attachment of the rectovaginal septum to the pelvic sidewall. Am J Obstet Gynecol. 2001; 185:41–43.
29. Nagata I, Murakami G, Suzuki D, Furuya K, Koyama M, Ohtsuka A. Histological features of the rectovaginal septum in elderly women and a proposal for posterior vaginal defect repair. Int Urogynecol J Pelvic Floor Dysfunct. 2007; 18:863–868.
30. Hirata E, Fujiwara H, Hayashi S, Ohtsuka A, Abe S, Murakami G, Kudo Y. Intergender differences in histological architecture of the fascia pelvis parietalis: a cadaveric study. Clin Anat. 2011; 24:469–477.
31. Takeyama M, Koyama M, Murakami G, Nagata I, Tomoe H, Furuya K. Nerve preservation in tension-free vaginal mesh procedures for pelvic organ prolapse: a cadaveric study using fresh and fixed cadavers. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:559–566.
32. Arakawa T, Murakami G, Nakajima F, Matsubara A, Ohtsuka A, Goto T, Teramoto T. Morphologies of the interfaces between the levator ani muscle and pelvic viscera, with special reference to muscle insertion into the anorectum in elderly Japanese. Anat Sci Int. 2004; 79:72–81.
33. Kinugasa Y, Murakami G, Suzuki D, Sugihara K. Histological identification of fascial structures posterolateral to the rectum. Br J Surg. 2007; 94:620–626.
34. Sato K, Sato T. The vascular and neuronal composition of the lateral ligament of the rectum and the rectosacral fascia. Surg Radiol Anat. 1991; 13:17–22.
35. Lin M, Chen W, Huang L, Ni J, Yin L. The anatomy of lateral ligament of the rectum and its role in total mesorectal excision. World J Surg. 2010; 34:594–598.
36. Açar HI, Kuzu MA. Important points for protection of the autonomic nerves during total mesorectal excision. Dis Colon Rectum. 2012; 55:907–912.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download