Abstract
A holy grail of curing neurodegenerative diseases is to identify the main causes and mechanisms underlying neuronal death. Many studies have sought to identify these targets in a wide variety of ways, but a more important task is to identify critical molecular targets and their origins. Potential molecular targets include advanced glycation end products (AGEs) that can promote neuronal cell death, thereby contributing to neurodegenerative disorders such as Alzheimer disease or Parkinson disease. In this study, we showed that AGE-albumin (glycated albumin) is synthesized in microglial cells and secreted in the human brain. Our results provide new insight into which microglial cells can promote the receptor for AGE-mediated neuronal cell death, eventually leading to neurodegenerative diseases.
We recently presented that albumin can be synthesized and secreted in human brain microglial cells[1]. We also showed that the synthesis and secretion of albumin from microglial cells is increased by microglial activation following exposure to lipopolysaccharide or amyloid β (Aβ) 1-42. However, the role of albumin remains unclear. Several studies have demonstrated that increased accumulation of advanced glycation end products (AGEs) in the brains of patients with Alzheimer disease (AD) suggests pathological roles for AGEs in neurodegenerative disorders, including AD[2-4]. Activated microglial cells and Aβ deposition have been colocalized with AGEs in the human brain[5, 6]. However, despite these reports, the origin, role, and detailed mechanisms underlying the involvement of AGEs in promoting neuronal cell death and neurodegeneration are poorly understood.
The aims of this study were to investigate whether AGEs in brain is glycated form of albumin from human microglial cells.
The immortalized human microglial cell line was used for in vitro studies. These cells were maintained at 37℃ under 5% CO2 in Dulbecco's modified Eagle's medium (DMEM with high glucose, Gibco, Calsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco) and 20 mg/ml gentamicin (Sigma-Aldrich, St. Louis, MO, USA). During experiments, human microglial cells were exposed to Aβ1-42 (Sigma-Aldrich) at concentrations ranging from 0 to 400 or 5 nM. The cells were then harvested for further analysis 6 hours after Aβ1-42 treatment.
Primary human neuronal cells were prepared from human brain tissues. The methods used for collection and use of brain tissue samples were approved by the Ethics Committee of the Seoul National University, College of Medicine, Seoul, Korea. Cells were dissociated from small pieces of human brain cortexes by incubating the tissue in phosphate-buffered saline (PBS) containing 0.25% trypsin and 40 mg/ml DNase I for 30 minutes at 37℃. Dissociated cells were then suspended in DMEM supplemented with 5% FBS, 5% horse serum, 20 mg/ml gentamicin, and 2.5 mg/ml amphotericin B (feeding medium), plated at a density of 106 cells/ml in 10-cm culture dishes (in 10-ml volume), and maintained at 37℃ in an incubator under 5% CO2/95% air atmosphere. Remaining neuronal cells were used for apoptosis-related characterization following treatment with AGE-albumin or albumin.
Cells were grown on Lab-Tek II slide chambers (Nunc, Naperville, IL, USA), rinsed with PBS, fixed in methanol for 15 minutes, and finally rinsed again with PBS. The fixed cells on slide chambers were incubated overnight at 4℃ with the following primary antibodies: rabbit anti-AGE antibody (1 : 200, Abcam, Cambridge, UK), mouse anti-human-albumin antibody (1 : 200, R&D Systems, Minneapolis, MN, USA). After overnight incubation, the cells were washed three times in PBS, and the slides were then incubated for 1 hour at room temperature with one of the following secondary antibodies: Alexa Fluor 633 anti-mouse IgG (1 : 500, Invitrogen, Carlsbad, CA, USA), Alexa Fluor 488 anti-rabbit IgG (1 : 500, Invitrogen), or Alexa Fluor 555 anti-goat IgG (1 : 500, Invitrogen). After washing the cells for three 10-minute intervals in PBS, cover slips were mounted onto glass slides by using Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA), and examined under a laser confocal fluorescence microscope (LSM-710, Carl Zeiss, Jena, Germany).
Adult Sprague-Dawley rats (230-350 g) were used in this study. The rats were maintained on a 12-hour light-dark cycle, had access to food and water ad libitum, and were acclimatized for at least 1 week prior to use.
Animals were anesthetized with ketamine HCl (0.75 mg/kg body weight) and xylazine (1 mg/kg body weight) prior to surgical procedures. For in vivo treatments, Aβ1-42 peptide was dissolved in sterile water at a concentration of 400 µM, and maintained at 4℃until use. Injection of Aβ1-42 into the entorhinal cortex (EC) was done with the aid of a stereotaxic instrument by tracing a midline incision into the scalp skin. The skull was pierced with a biological electric drill at the bregma (posteriorly, 8.3 mm; laterally, 5.4 mm), and the 30-gauge needle on a 5-µl Hamilton syringe was lowered vertically until it reached the target areas (depth, 4.5 mm). A total of 5 µl of 200 µM Aβ1-42 solution or 5 µl of PBS was injected gradually, at a rate of 1 µl/min, with an automatic microinjector. The syringe was then slowly removed, and the surgical incisions were sutured with wound clips followed by topical treatment with antibiotics.
Most rats were allowed to recover for a total of 3 days post injection. After full recovery, all rats were re-anesthetized in the same manner, and perfused transcardially with 100-200 ml of heparinized saline at 18℃ followed by 400 ml of 4% paraformaldehyde-lysine periodate in 0.1 M sodium phosphate buffer (pH 7.4). The brains were removed, placed in the same fixative for 4 hours at 4℃, and then, transferred into ice-cold 0.1 M PBS containing 20% sucrose. The brains were then cut in a transverse plane at 30-µm thickness with a frozen microtome, and then stored at -80℃ until use.
PLA was performed in both primary cells and brain tissues to visualize the extent (frequency) of protein-protein interactions. Target tissues were washed in ice-chilled PBS and incubated overnight with mouse anti-human-AGE antibody (1 : 200, R&D Systems) and rabbit anti-Aβ antibody (1 : 200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4℃. Hoechst and PLA staining was performed using the Duolink Detection Kit (Life Technology, Pittsburg, PA, USA) according to the manufacturer's protocol. Tissue specimens were mounted with Vectashield mounting media (Vector Laboratories) and analyzed using an LSM 710 confocal microscope (Zeiss). The number of in situ PLA signals per cell was counted using the semi-automated image analysis program BlobFinderV3.0.
Whole cell lysates were prepared with RIPA buffer containing 4% CHAPS. Proteins from each group were separated in 4-12% polyacrylamide gels (Life Technology) and transferred to nitrocellulose membranes. The primary antibodies used were: anti-receptor protein for AGEs (RAGE; 1 : 200, Santa Cruz), and anti β-actin (1 : 1,000, Cell Signaling Technology, Danvers, MA, USA).
The densitometric intensity of each immunoreactive band was determined using a gel digitizing Imagepro software. The data summarized in this report are from at least 3 independent experiments, unless stated otherwise. Statistical analyses were performed using the Student's t-test, and P<0.05 was considered statistically significant.
We first investigated the distribution of AGE and albumin in the human microglial cell line and human primary microglial cells, respectively. Surprisingly, most AGEs were co-localized with albumin, suggesting that AGE-albumin could be a major AGE in brain microglial cells (Fig. 1).
Furthermore, to demonstrate the co-localization of AGE-albumin and the activated microglial cell marker Iba-1, we performed triple immunohistochemical staining in Aβ-treated human microglial cells and primary human microglial cells. The expression levels of AGE were markedly increased following Aβ exposure, and most AGE co-localized with albumin in the human microglial cells and human primary microglial cells. In addition, AGE-albumin and Iba-1 expression levels were strikingly elevated, and they colocalized in Aβ-exposed rat brains (Fig. 1).
Relationship between AGE-albumin and Aβ contributing to Aβ aggregation was studied. Amyloid plaques in Aβ-treated rat brains and human AD brains were revealed as increased by triple-labeled fluorescent microscopic images (Fig. 2). Proximity ligation O-Link analysis was performed by fluorescent microscopic images to determine the relative extents of interaction between albumin and Aβ in human microglial cells and rat brains before and after Aβ treatment, as well as human brains from AD individuals, respectively. Interaction between AGE-albumin and Aβ, contributing to increased synthesis and aggregation of Aβ were increased after Aβ treatment (Fig. 2).
Proximity ligation assay data showed that the amount of AGE-RAGE interaction was significantly increased in Aβ-exposed rat brain neurons (Fig. 3) compared to the neurons from untreated rat brains.
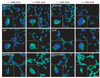
Since stress-activated mitogen-activated protein kinases (MAPKs) are critical in initiating apoptosis[7, 8], we monitored changes in the levels of MAPKs in human primary neurons. Immunohistochemistry analysis showed that, relative to the untreated controls, the levels of p38K, p-p38K, stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK), and p-SAPK/JNK were significantly increased, while the level of phosphorylated extracellular signal-regulated kinases 1 and 2 (pERK1/2) was de creased after human primary neurons were exposed to AGE-albumin (Fig. 4).
On the basis of observed albumin synthesis in microglial cells[1], co-localization of AGEs with Aβ deposits in AD brains[2-4], and detection of elevated AGEs[5, 6], we hypothesized that AGE-albumin, synthesized in and secreted from microglial cells, promotes neuronal cell death. To test this hypothesis and to further study the mechanism by which AGE-albumin promotes neuronal cell death, we first investigated the distribution of AGE and albumin in the human microglial cell line and human primary microglial cells, respectively. Surprisingly, most AGEs were co-localized with albumin, suggesting that AGE-albumin could be a major AGE in brain microglial cells. Furthermore, to demonstrate the co-localization of AGE-albumin and the activated microglial cell marker Iba-1, we performed triple immunohistochemical staining in Aβ-treated human microglial cells and primary human microglial cells. The expression levels of AGE were markedly increased following Aβ exposure. In addition, AGE-albumin and Iba-1 expression levels were strikingly elevated, and they co-localized in Aβ-exposed rat brains. On the basis of these results, we concluded that AGE-albumin, the most abundant and modified AGE-protein, is produced largely in the microglial cells of Aβ-exposed human and rat brains.
Relationship between AGE-albumin and Aβ contributing to Aβ aggregation was studied. Proximity ligation O-Link analysis was revealed that the interaction between AGE-albumin and Aβ, contributing to increased synthesis and aggregation of Aβ were increased after Aβ treatment.
Increased expression of the neuronal RAGE is highly correlated with neuronal death and the development and progression of AD[3]. In addition, AGE binds to RAGE in primary neurons[4]. Therefore, we also evaluated whether AGE-albumin could increase RAGE production, which would strongly indicate neuronal apoptosis in AD[3, 9], leading to cell death in human primary neurons.
Immunohistochemistry analysis showed that, relative to the untreated controls, the levels of p38K, p-p38K, SAPK/JNK, and p-SAPK/JNK were significantly increased, while the level of pERK1/2 was decreased after human primary neurons were exposed to AGE-albumin. These data indicate that AGE-albumin directly activates MAPK pathway, as demonstrated previously[10, 11].
In summary, our current data show that AGEs are mostly AGE-albumin in brain. Activated human microglial cells produce and secrete AGE-albumin, which promotes increased RAGE in neurons. Our results, therefore, provide new insight into how microglial cells playing an important role in promoting neuronal cell death may contribute to neurodegeneration.
Figures and Tables
Fig. 1
Distribution and synthesis of advanced glycation end products (AGE)-albumin in human microglial cells and rat brain. Triple-labeled confocal microscopy was used to study the distribution and relative levels of albumin (ALB, green), AGE (red) and a specific marker of microglial cells (Iba1, blue) in the human microglial cell line, human primary microglial cells, and entorhinal cortex of rat brain after amyloid β (Aβ) treatment. Scale bar=50 µm.
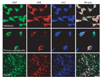
Fig. 2
Relationship between advanced glycation end products (AGE)-albumin (ALB) and amyloid β (Aβ) contributing to Aβ aggregation. (A) Co-localization of albumin (blue), AGE (red), and ThT fluorescence (green) in amyloid plaques in Aβ-treated rat brains and human Alzheimer disease (AD) brains were evaluated by triple-labeled fluorescent microscopic images. (B) In teraction between AGE-ALB and Aβ, contributing to increased synthesis and aggregation of Aβ. Proximity ligation O-Link analysis was performed by fluorescent microscopic images to determine the relative extents of interaction (red spots) between ALB and Aβ in human microglial cells and rat brains before and after Aβ treatment, as well as human brains from normal and AD individuals, respectively. The average numbers of blob per cell from each sample are compared in bar graphs. Scale bars=50 µm.
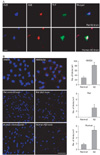
Fig. 3
Relative changes in advanced glycation end products (AGE) and receptor protein for AGEs (RAGE) interactions. (A) Proximity ligation assay was performed using fluorescent microscopy images to determine the relative extents of interaction (red spots) between AGE and RAGE in the rat brains before or after amyloid β (Aβ) treatment. Scale bar=50 µm. (B) The average number of blobs per cell from each sample is summarized in bar graphs.
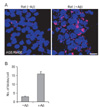
Fig. 4
Induction of the neuronal mitogen-activated protein kinases (MAPK) pathway by advanced glycation end products (AGE)-albumin (ALB). Double confocal microscopy images simultaneously show relative levels of extracellular signal-regulated kinases 1 and 2 (ERK1/2), pERK1/2, p38K, pp38K, stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK), pSAPK/JNK (green), or DAPI (blue) in human primary neuronal cells before and after AGE-ALB treatment for 6 h. Scale bar=50 µm.
Acknowledgements
The authors are grateful to Dr. Tadashi Yamamoto (Niigata University, Japan) for providing human brain specimens from AD and normal individuals. This work was supported by the National Research Foundation grant funded by the Korean government (MEST) (No. 2010-0002054 and SC-2110).
References
1. Ahn SM, Byun K, Cho K, Kim JY, Yoo JS, Kim D, Paek SH, Kim SU, Simpson RJ, Lee B. Human microglial cells synthesize albumin in brain. PLoS One. 2008. 3:e2829.
2. Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001. 108:949–955.
3. Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996. 382:685–691.
4. Yan SD, Bierhaus A, Nawroth PP, Stern DM. RAGE and Alzheimer's disease: a progression factor for amyloid-beta-induced cellular perturbation? J Alzheimers Dis. 2009. 16:833–843.
5. Schumacher U, Kretzschmar H, Pfüller U. Staining of cerebral amyloid plaque glycoproteins in patients with Alzheimer's disease with the microglia-specific lectin from mistletoe. Acta Neuropathol. 1994. 87:422–424.
6. Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, Hammers A, Tai YF, Fox N, Kennedy A, Rossor M, Brooks DJ. Microglia, amyloid, and cognition in Alzheimer's disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008. 32:412–419.
7. Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003. 4:552–565.
8. Hajnóczky G, Csordás G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006. 40:553–560.
9. Takeuchi M, Sato T, Takino J, Kobayashi Y, Furuno S, Kikuchi S, Yamagishi S. Diagnostic utility of serum or cerebrospinal fluid levels of toxic advanced glycation end-products (TAGE) in early detection of Alzheimer's disease. Med Hypotheses. 2007. 69:1358–1366.
10. Ko HW, Park KY, Kim H, Han PL, Kim YU, Gwag BJ, Choi EJ. Ca2+-mediated activation of c-Jun N-terminal kinase and nuclear factor kappa B by NMDA in cortical cell cultures. J Neurochem. 1998. 71:1390–1395.
11. Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006. 281:21256–21265.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download