Abstract
Objective
Hyperuricemia is known as a risk factor that causes and worsens kidney diseases through a variety of mechanisms. Recent animal studies reported that the cor-rection of hyperuricemia improved the renal function, but there have been few human studies. This study examined whether a hypouricemic treatment affects the renal function in Korean patients with gout.
Methods
Two hundred sixty-seven gout patients who were prescribed uric acid lowering agents for more than 1 year were enrolled at the Division of Rheumatology in the National Health Insurance Corporation Ilsan Hospital and Yonsei University Severance Hospital from January 2005 to January 2010. The following were examined: the levels of serum uric acid and serum creatinine, the amount of 24-hour urine uric acid, glomerular filtration rate (GFR), and abdominal ultrasound findings at baseline and followup.
Results
Mean age of the study subjects was 54.4±13.9 years. Two hundred forty-seven patients were male and 20 patients were female. The mean treatment duration was 35.0±19.5 months. Among the 267 patients, 219 and 19 patients received monotherapy with allopurinol and benzbromarone respectively, and 29 patients received combination therapy with allopurinol and benzbromarone. After the treatment with uric acid lowering agents, the serum uric acid and creatinine levels decreased significantly (8.05±1.96 mg/dL vs 6.16±1.46 mg/dL, p<0.001, 1.25±0.46 mg/dL vs 1.18±0.42 mg/dL, p=0.001, respectively) and the GFR increased significantly (74.4±27.0 mL/min/1.73 m3 vs 80.2±31.6 mL/min/1.73 m3, p<0.001).
References
1. Marc CH, Alan JS, Josef SS, Michal EW, Michael HW. Rheumatology. 4th ed.p. 1805–34. London: Mosby;2008.
2. Harris CM, Lloyd DC, Lewis J. The prevalence and prophylaxis of gout in England. J Clin Epidemiol. 1995; 48:1153–8.
3. Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008; 58:26–35.
4. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–52.
5. Rho YH, Choi SJ, Lee YH, Ji JD, Choi KM, Baik SH, et al. The prevalence of metabolic syndrome in patients with gout: a multicenter study. J Korean Med Sci. 2005; 20:1029–33.
6. Vaziri ND, Freel RW, Hatch M. Effect of chronic experimental renal insufficiency on urate metabolism. J Am Soc Nephrol. 1995; 6:1313–7.
7. Syrjänen J, Mustonen J, Pasternack A. Hypertriglycer-idaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant. 2000; 15:34–42.
8. Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M. Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron. 2001; 87:333–9.
9. Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G. Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest. 2001; 31:318–21.
10. Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001; 24:691–7.
11. Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002; 13:2888–97.
12. Sánchez-Lozada LG, Tapia E, Santamaría J, Avila-Casado C, Soto V, Nepomuceno T, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005; 67:237–47.
13. Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003; 41:1183–90.
14. Nakagawa T, Mazzali M, Kang DH, Kanellis J, Watanabe S, Sanchez-Lozada LG, et al. Hyperuricemia causes glomerular hypertrophy in the rat. Am J Nephrol. 2003; 23:2–7.
15. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977; 20:895–900.
16. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003; 139:137–47.
17. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984; 76:47–56.
18. Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007; 57:109–15.
19. Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005; 28:1769–78.
20. Berger L, Yü TF. Renal function in gout. IV. An analysis of 524 gouty subjects including longterm followup studies. Am J Med. 1975; 59:605–13.
21. Talbott JH, Terrlan KL. The kidney in gout. Medicine (Baltimore). 1960; 39:405–67.
22. Daniel EW, Hocine T, Essam FE, John LG, Deeb NS, Andrew SL. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008; 19:1207–11.
23. Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003; 41:1287–93.
24. Harris RC, Breyer MD. Physiological regulation of cyclo-oxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001; 281:F1–11.
25. Young W, Mahboubi K, Haider A, Li I, Ferreri NR. Cyclooxygenase-2 is required for tumor necrosis factor-alpha- and angiotensin II-mediated proliferation of vascular smooth muscle cells. Circ Res. 2000; 86:906–14.
26. Desco MC, Asensi M, Márquez R, Martínez-Valls J, Vento M, Pallardó FV, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes. 2002; 51:1118–24.
27. Sánchez-Lozada LG, Tapia E, Avila-Casado C, Soto V, Franco M, Santamaria J, et al. Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol Renal Physiol. 2002; 283:F1105–10.
28. Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001; 38:1101–6.
29. Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriol-opathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002; 282:F991–7.
30. Saito I, Saruta T, Kondo K, Nakamura R, Oguro T, Yamagami K, et al. Serum uric acid and the renin- angiotensin system in hypertension. J Am Geriatr Soc. 1978; 26:241–7.
31. Perez-Ruiz F, Calabozo M, Herrero-Beites AM, García-Erauskin G, Pijoan JI. Improvement of renal function in patients with chronic gout after proper control of hyperuricemia and gouty bouts. Nephron. 2000; 86:287–91.
32. Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006; 47:51–9.
33. Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, et al. British Society for Rheumatology and British Health Professionals in Rheumatology Standards, Guidelines and Audit Working Group (SGAWG). British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology (Oxford). 2007; 46:1372–4.
34. Perez-Ruiz F, Alonso-Ruiz A, Calabozo M, Herrero-Beites A, García-Erauskin G, Ruiz-Lucea E. Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis. 1998; 57:545–9.
Figure 1.
The changes in the serum uric acid levels were correlated with the changes in the serum creatinine levels after the treatment with hypouricemic agents (r=0.18, p=0.003).
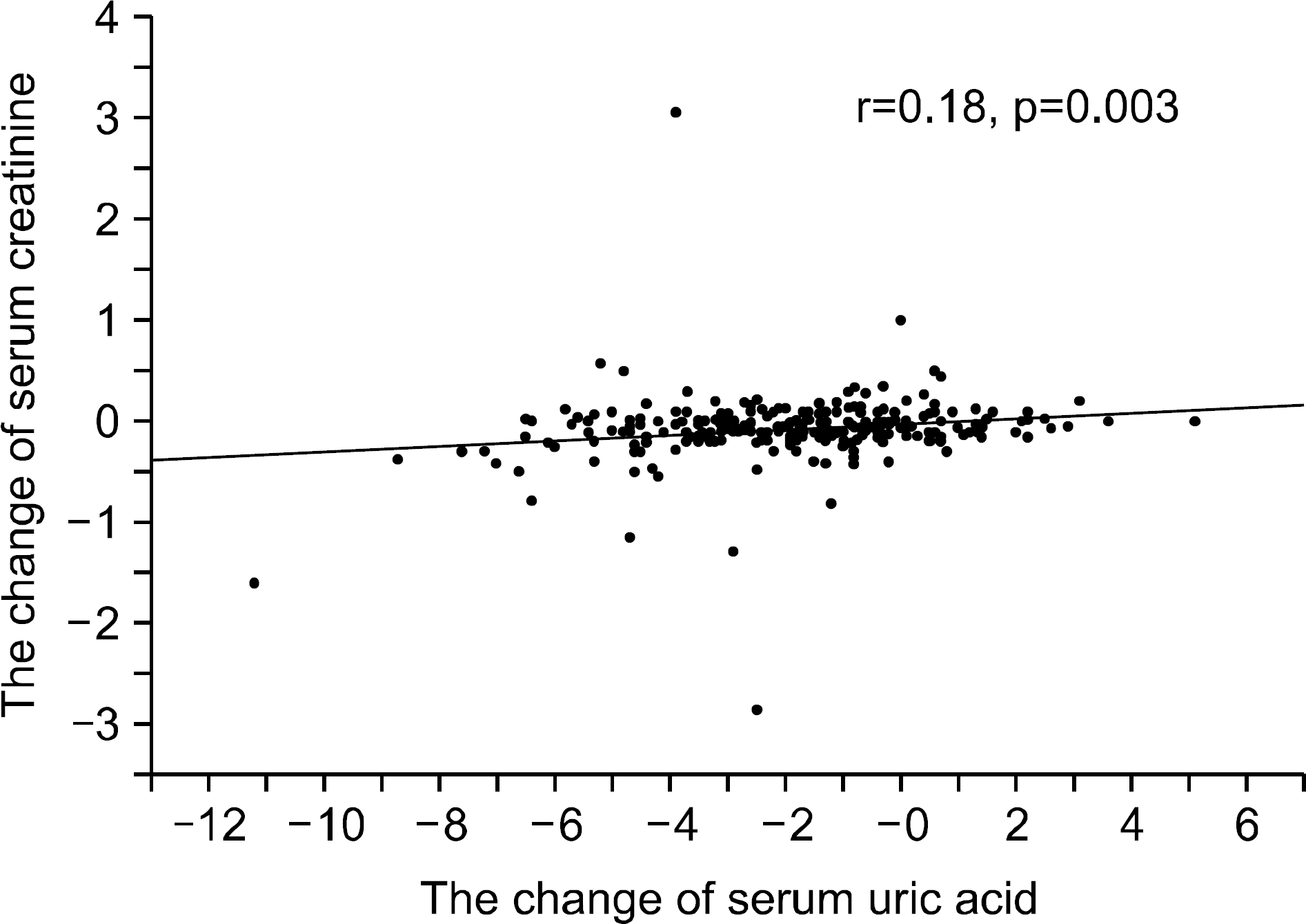
Figure 2.
The changes of serum uric acid levels were correlated with the changes in the creatinine clearance levels after the treatment with hypouricemic agents (r=−0.25, p<0.001).

Table 1.
Characteristics of the gout patients
Table 2.
Changes in the serum uric acid, serum creatinine and creatinine clearance in 267 gout patients after treatment with the hypouricemic agents (N, (%))
| Serum UA | Serum Cr | CCr | |
|---|---|---|---|
| Improved | 223 (83.5) | 174 (65.2) | 164 (61.4) |
| Deteriorated | 42 (15.7) | 62 (23.2) | 88 (33) |
| Not changed | 2 (0.7) | 31 (11.6) | 15 (5.6) |
Table 3.
Creatinine clearance, serum uric acid, and serum creatinine in 267 gout patients before and after treatment with the hypouricemic agents
| Before treatment | After treatment | p-value | |
|---|---|---|---|
| CCr (mL/min/1.73 m3) | 74.41±27.02 | 80.18±31.55 | <0.001 |
| Serum UA (mg/dL) | 8.05±1.96 | 6.16±1.46 | <0.001 |
| Serum Cr (mg/dL) | 1.25±0.46 | 1.18±0.42 | 0.001 |
Table 4.
Significance of the random effects in mixed model analysis∗
| Variance-covariance estimates (SE) of random effects | ||||
|---|---|---|---|---|
| Serum Cr | p | CCr | p | |
| Serum UA | 0.04 (0.01) | <0.001 | −3.1 (0.68) | <0.001 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download