Abstract
Food-dependent exercise-induced anaphylaxis (FDEIA) is a type of exercise-induced anaphylaxis associated with postprandial exercise. We describe a 19-year-old man with FDEIA. Our patient complained of urticaria, angioedema, dizziness and hypotension associated with exercise after ingestion of walnut-containing foods in a warm environment. Skin prick test and prick to prick test were positive for walnut antigen. The attack didn't occur by free running outside for 10 min 2 h after taking walnuts, and the temperature was about -2℃. Food-exercise test was done again in a warm environment based on prior history. Anaphylaxis was developed after exercise for 10 min in a warm environment after taking walnuts. Some environmental factors such as high temperature and high humidity or cold temperature may influence exercise-induced anaphylaxis. In our case, the cofactor was a warm environment: the challenge test done in a cold environment was negative, but positive in a warm environment. Physicians should be aware that the challenge test of FDEIA can show different results depending on temperature.
Anaphylaxis is defined as a severe, life-threatening, generalized or systemic hypersensitivity reaction involving several organs, particularly the skin, respiratory tract, gastrointestinal tract, and cardiovascular system [1]. The prevalence of anaphylaxis is estimated to be 0.014% to 2% [2, 3]. The causes of anaphylaxis were drugs, food, insect stings, latex, radiocontrast media, physical factors such as exercise, cold, heat, sunlight/UV radiation, and idiopathic [3, 4].
Food-dependent exercise-induced anaphylaxis (FDEIA) is a type of exercise-induced anaphylaxis (EIA) that occurs only when a sensitized individual ingests food allergens and proceeds to exercise within 2 to 6 h [5]. Physical exercise without ingestion of the causative food, as well as ingestion of food allergens without postprandial exercise, is well tolerated [6]. Food-exercise challenge test can be performed for a diagnosis of FDEIA based on the association between the food allergen and physical activity.
In this study we report a case of FDEIA, in whom anaphylactic events occurred after challenge test in a warm environment, but not in a cold environment.
A 19-year-old man was referred to the asthma and allergy clinic in the Seoul National University Bundang Hospital after being hospitalized due to sudden urticaria, angioedema, dizziness and hypotension (systolic blood pressure was 80 mmHg). A thorough history taking revealed that these episodes occurred when the patient exercised (free running) 4 h after eating walnut cookies in a warm day in early September.
He denied any allergic diseases. In order to confirm the diagnosis and evaluate the causative factors, skin prick test and provocation tests were performed.
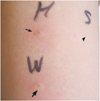
Complete physical examination was unremarkable. He underwent skin prick test, the result was positive for walnuts (4 × 4 mm for histamine, 4 × 4 mm for walnuts). Prick to prick test for walnuts was also positive (4 × 4 mm for histamine, 6 × 5 mm for walnuts, Fig. 1). An immunological evaluation demonstrated positive UniCAP to peanuts (class 1), almonds (class 2) and walnuts (class 3).
In oral challenge test for walnuts of 15 g, he tolerated except of oral itching, which showed that he had an oral allergy syndrome to walnuts. Exercise provocation test, free running for 10 min, was done on the other day and the result was negative. On another day, he took walnuts of 15 g and proceeded to free running for 10 min outside after 2 h. Only a few hives on neck and elbow were seen 2 h later. Although a diagnosis of food-dependent exercise-induced mild urticaria was established, anaphylactic events did not occur. We noticed that this food-exercise challenge was done in a cold environment (temperature of about -2℃), but prior events were induced in a warm environment (temperature of about 26℃). Oral food challenge test and exercise provocation test performed independently in a warm environment were negative. So he took 10 min exercise 2 h after walnut ingestion in a warm environment. Hives were seen on neck 15 min later and whole body 1 h later. After 2 h, angioedema on face and neck was developed and he appealed dyspnea (Fig. 2). Stridor was developed around the trachea. After injection of epinephrine, chlorpheniramine and hydrocortisone, he was recovered.
The patient was discharged with a prescription for EpiPen® (Dey, USA). He was also advised to avoid walnut-containing products for several h prior to exertion.
In this study, we report a case of FDEIA presented only in a warm environment, not in a cold environment. Oral food challenge test, exercise provocation test and food-exercise challenge test to confirm the diagnosis were negative. But we noticed that prior events occurred in a warm environment and this food-exercise challenge test was performed in a cold environment. So we repeated food-exercise challenge test in a warm environment and anaphylactic events were occurred. In our patient, the cofactor influencing the result of FDEIA was a warm environment.
FDEIA is described of anaphylaxis occurring after exercise preceded by ingestion of the causative foods. Maulitz et al. [7] reported the first case of anaphylaxis triggered by food-dependent exercise in 1979. Their patient experienced anaphylactic events induced by shellfish intake 5 and 24 h prior two exercise-related events. Kidd and coworkers [8] designed the phenomenon of FDEIA as a syndrome characterized by hives, cutaneous itching, dizziness, weakness, abdominal cramping, and angioedema associated with certain foods and exercise.
The prevalence of FDEIA is not well-documented. Yang et al. [3] reported the prevalence of anaphylaxis was 0.014% and 13.2% of anaphylaxis was FDEIA. The others reported the incidence of anaphylaxis was 0.5-2% [2] and that of FDEIA was 0.017% [9].
For the diagnosis of FDEIA, the first step is to take a careful history to determine if there are other reasons for the symptoms. This is followed by skin prick test or in vitro serum specific IgE assays [10, 11]. Open food-exercise challenge or double-blinded, placebo-controlled food-exercise challenge should be performed to confirm the diagnosis of FDEIA [10].
The pathophysiology of FDEIA remains to be elucidated. FDEIA may occur as a result of increased histamine release from basophils activated by exercise-induced, transient serum hyperosmolarity after exposure to sensitized food allergens [12]. Skin mast cell degranulation from skin biopsies of patients with EIA during anaphylactic events was reported [13]. Alterations in serum pH after prolonged and strenuous exercise may trigger mast cell degranulation [12]. Moreover, vigorous exercise facilitates allergen absorption from the gastrointestinal track, leading to FDEIA [14]. Tissue transglutaminase (tTG) activity in the intestinal mucosa could be activated by exercise, and may result in the formation of peptide aggregation that facilitates greater IgE cross-linking [15]. Omega-5 gliadin, a major allergen in FDEIA to wheat, could be cross-linked by tTG [16].
Robson-Ansley and Toit [12] hypothesized that the triggering factor for FDEIA is exercise-induced redistribution of blood flow from the viscera to the skeletal muscle and skin. Redistribution of blood results in displacement of the ingested allergens from the gut to the skin or skeletal muscle [12]. Mucosal mast cells and connective tissue mast cells are phenotypically different because mast cells invade the connective and mucosal tissue as mast cell progenitors. Two phenotypes of mast cell have biochemical differences and differ in their quantitative release of histamine and metabolism of arachidonic acid to leukotriene C4 and B4 [17]. Absorbed food allergens are tolerated by mucosal mast cells, thus provoke no symptoms at rest. Mast cell in the connective tissue could increase the potential for anaphylaxis.
External cofactors eliciting EIA, in addition to food, are warm temperature, cold temperature and high humidity [16]. In this case, warm temperature is considered to be related to FDEIA than cold temperature while it has been previously reported that most of external cofactor for FDEIA were 'cold' [16, 18]. This report is the first case of 'heat-related' FDEIA in Korea. Our patient did not experience FDEIA in a cold environment. It is possible that redistribution of blood flow to connective tissue was not enough to develop anaphylaxis because of the cold temperature. This could be explained that patients experiencing FDEIA in generally develop cutaneous warmth and pruritis followed by cardiorespiratory collapse and airway compromise, but the full evolution of the syndrome may be halted if the trigger is insufficient [19]. So the triggering factors of our patient could be insufficient to raise enough body temperature in a cold environment. Also individual variation in the threshold of mast cells to heat exposure could influence the results.
Multiple triggering factors can develop FDEIA. We must consider possible other triggering factors when the challenge test does not reproduce the symptoms of FDEIA. Physicians should be aware that the challenge test of FDEIA can show different results depending on temperature.
Figures and Tables
References
1. Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, Kowalski ML, Mygind N, Ring J, van Cauwenberge P, van Hage-Hamsten M, Wüthrich B. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001; 56:813–824.

2. Lieberman P, Camargo CA Jr, Bohlke K, Jick H, Miller RL, Sheikh A, Simons FE. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006; 97:596–602.

3. Yang MS, Lee SH, Kim TW, Kwon JW, Lee SM, Kim SH, Kwon HS, Park CH, Park HW, Kim SS, Cho SH, Min KU, Kim YY, Chang YS. Epidemiologic and clinical features of anaphylaxis in Korea. Ann Allergy Asthma Immunol. 2008; 100:31–36.

5. Wong CG, Mace SR. Food-dependent exercise-induced anaphylaxis: a case related to chickpea ingestion and review. Allergy Asthma Clin Immunol. 2007; 3:134–137.

6. Wolańczyk-Medrala A, Barg W, Radlińska A, Panaszek B, Medrala W. Food-dependent exercise-induced anaphylaxis-sequence of causative factors might be reversed. Ann Agric Environ Med. 2010; 17:315–317.
7. Maulitz RM, Pratt DS, Schocket AL. Exercise-induced anaphylactic reaction to shellfish. J Allergy Clin Immunol. 1979; 63:433–434.

8. Kidd JM 3rd, Cohen SH, Sosman AJ, Fink JN. Food-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 1983; 71:407–411.
9. Aihara Y, Takahashi Y, Kotoyori T, Mitsuda T, Ito R, Aihara M, Ikezawa Z, Yokota S. Frequency of food-dependent, exercise-induced anaphylaxis in Japanese junior-high-school students. J Allergy Clin Immunol. 2001; 108:1035–1039.

10. Kurowski K, Boxer RW. Food allergies: detection and management. Am Fam Physician. 2008; 77:1678–1686.
11. Yang MS, Lee SH, Kim KM, Kwon HS, Kim DI, Park CH, Son SW, Park HW, Chang YS, Kim SS, Cho SH, Min KU, Kim YY. A case report of food-dependent exercise-induced anaphylaxis to apples. Korean J Asthma Allergy Clin Immunol. 2006; 26:242–245.
12. Robson-Ansley P, Toit GD. Pathophysiology, diagnosis and management of exercise-induced anaphylaxis. Curr Opin Allergy Clin Immunol. 2010; 10:312–317.

13. Sheffer AL, Tong AK, Murphy GF, Lewis RA, McFadden ER Jr, Austen KF. Exercise-induced anaphylaxis: a serious form of physical allergy associated with mast cell degranulation. J Allergy Clin Immunol. 1985; 75:479–484.

14. de Oliveira EP, Burini RC. Food-dependent, exercise-induced gastrointestinal distress. J Int Soc Sports Nutr. 2011; 8:12.

15. Palosuo K, Varjonen E, Nurkkala J, Kalkkinen N, Harvima R, Reunala T, Alenius H. Transglutaminase-mediated cross-linking of a peptic fraction of omega-5 gliadin enhances IgE reactivity in wheat-dependent, exercise-induced anaphylaxis. J Allergy Clin Immunol. 2003; 111:1386–1392.
16. Barg W, Medrala W, Wolanczyk-Medrala A. Exercise-induced anaphylaxis: an update on diagnosis and treatment. Curr Allergy Asthma Rep. 2011; 11:45–51.

17. Welle M. Development, significance, and heterogeneity of mast cells with particular regard to the mast cell-specific proteases chymase and tryptase. J Leukoc Biol. 1997; 61:233–245.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download