Abstract
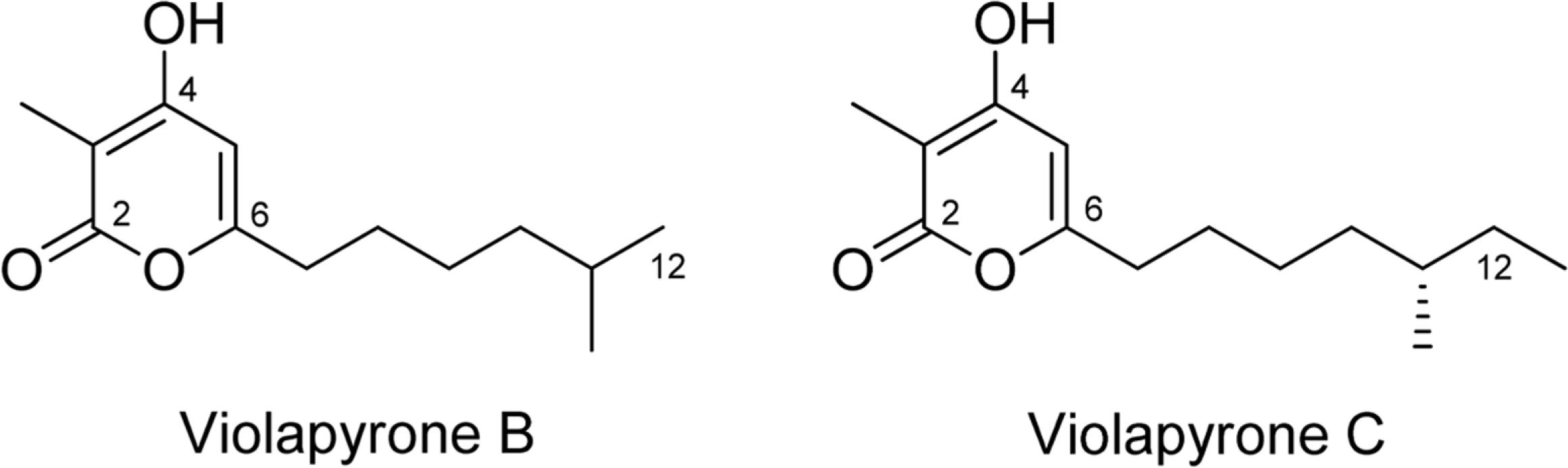
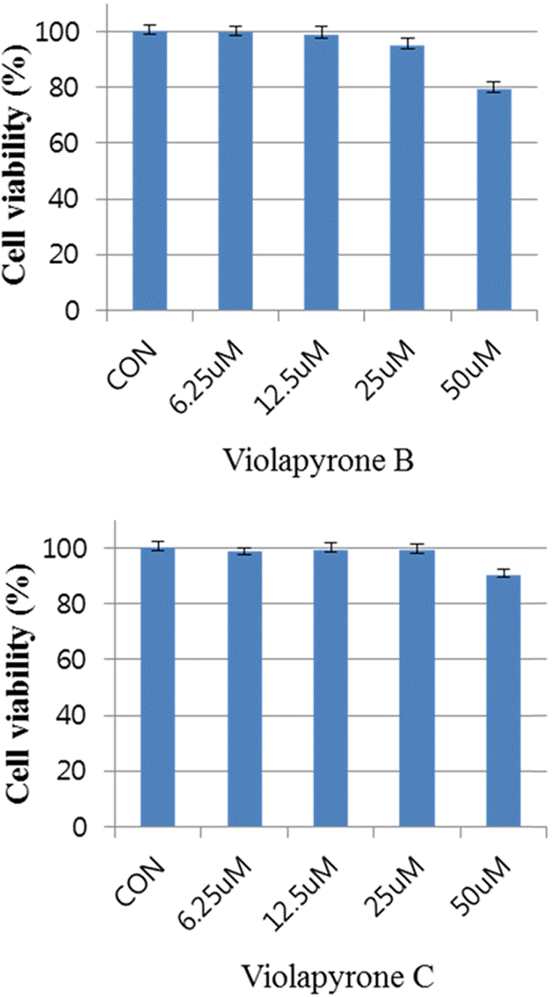
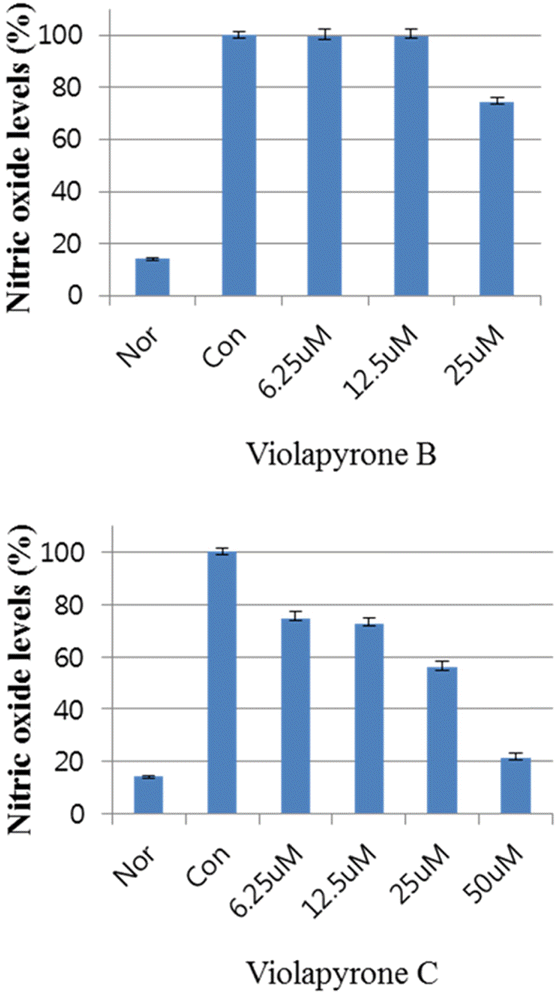
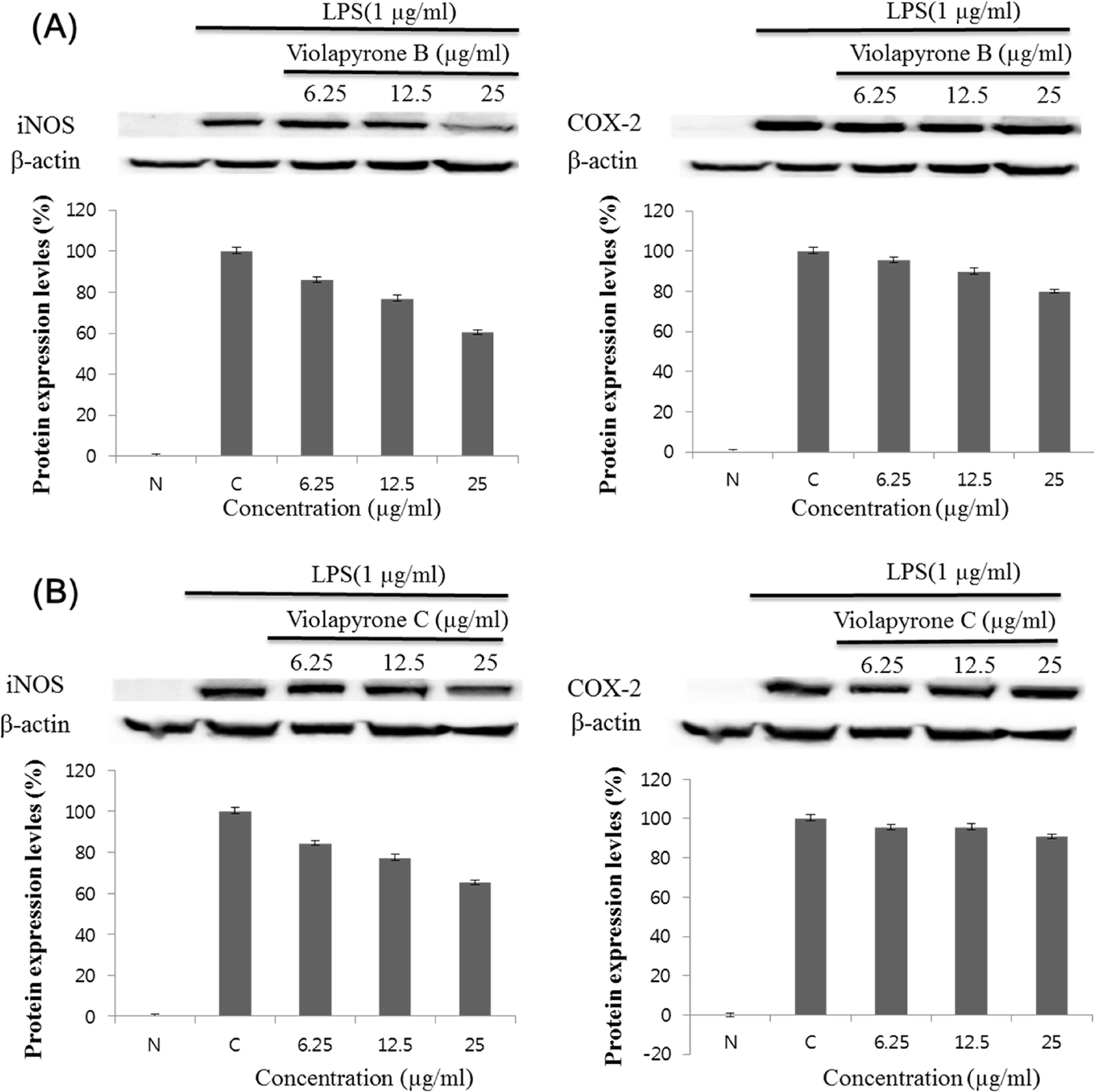
Recently, we reported violapyrones B, C, H and I, unusual 3, 4, 6-trisubstituted α-pyrone derivatives, from the culture broth of the marine Streptomyces sp. 112CH148. In previous studies, violapyrones have been shown to have antibacterial and antitumor activities. However, the anti-inflammatory effect of violapyrones has not been reported yet. As part of our ongoing study for the discovery of bioactive metabolites from marine microorganisms, we found that violapyrones also have anti-inflammatory activity. In this study, we investigated the effect of violapyrones on LPS-induced inflammatory responses in vitro. Violapyrones B and C did not affect the viability of RAW 264.7 cells at concentrations up to 25 μM. However, violapyrones B and C inhibited the production of NO compared to the LPS-induced control. In addition, violapyrones B and C down-regulated the expression of iNOS protein in LPS-stimulated RAW 264.7 cells. To the best of our knowledge, this is the first report on the anti-inflammatory activity of violapyrones B and C.
REFERENCES
(1). Ferrero-Miliani L., Nielsen O. H., Andersen P. S., Girardin S. E.Clin. Exp. Immunol. 2007; 147:227–235.
(2). Moncada S., Palmer R. M., Higgs E. A.Pharmacol. Rev. 1991; 43:109–142.
(3). Azad N., Rojanasakul Y., Vallyathan V. J.Toxicol. Environ. Health B Crit. Rev. 2008; 11:1–15.
(4). Libby P.Nature. 2002; 420:868–874.
(5). Tracey K. J.Nature. 2002; 420:853–859.
(6). Weninger S. C., Yankner B. A.Nat. Med. 2001; 7:527–528.
(7). Posadas I., Terencio M. C., Guillén I., Ferrándiz M. L., Coloma J., Payá M., Alcaraz M.Naunyn Schmiedebergs Arch. Pharmacol. 2000; 361:98–106.
(8). Huang G. J., Pan C. H., Liu F. C., Wu T. S., Wu C. H.Food Chem. Toxicol. 2012; 50:1485–1493.
(9). Lee J. S., Shin J., Shin H. J., Lee H. S., Lee Y. J., Lee H. S., Won H.Eur. J. Org. Chem. 2014; 21:4472–4476.
(10). Shin H. J., Lee H. S., Lee J. S., Shin J., Lee M. A., Lee H. S., Lee Y. J., Yun J., Kang J. S.Mar. Drugs. 2014; 12:3283–3291.
(11). Zhang J., Jiang Y., Cao Y., Liu J., Zheng D., Chen X., Han L., Jiang C., Huang X. J.Nat. Prod. 2013; 76:2126–2130.
(12). Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B.Cancer Res. 1987; 47:936–942.
(13). Kim H. J., Kim D. H., Lee J. Y., Hwang J. S., Lee J. H., Lee S. G., Jeong H. G., An B. J. J.Life Sci. 2013; 23:38–43.
(14). Shen B.Curr. Opin. Chem. Biol. 2003; 7:285–295.
(15). Sugiyama Y., Oya A., Kudo T., Hirota A. J.Antibiot. 2010; 63:365–369.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download