Abstract
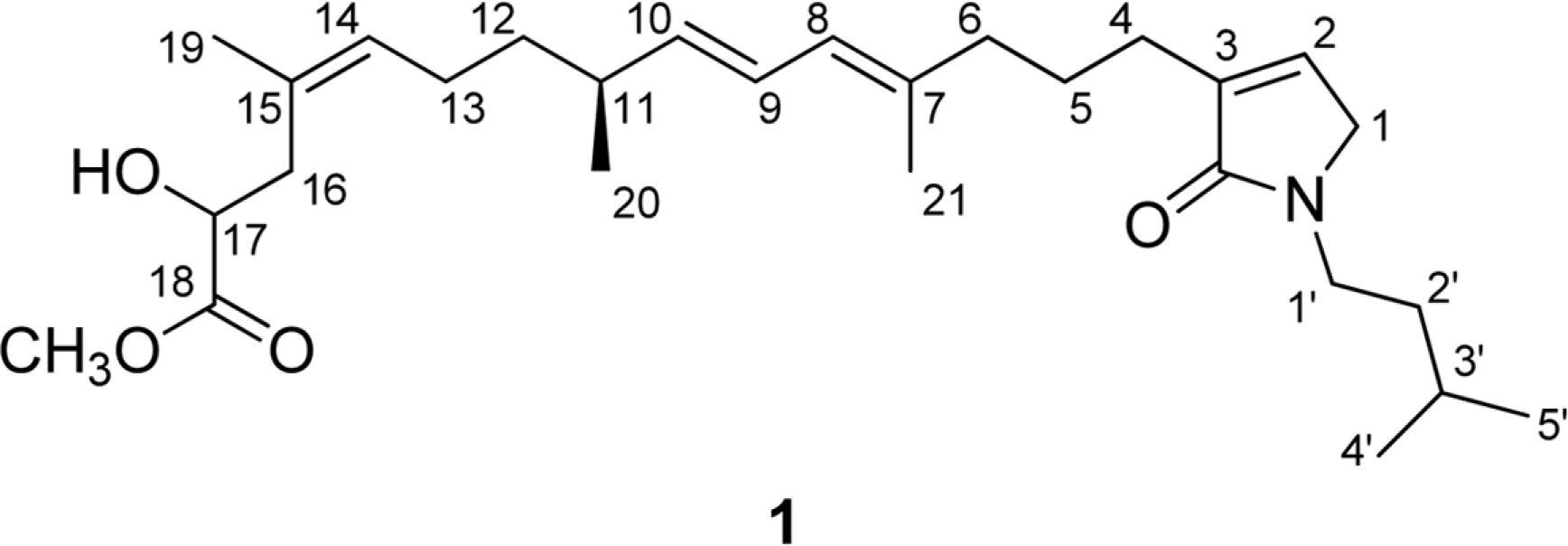
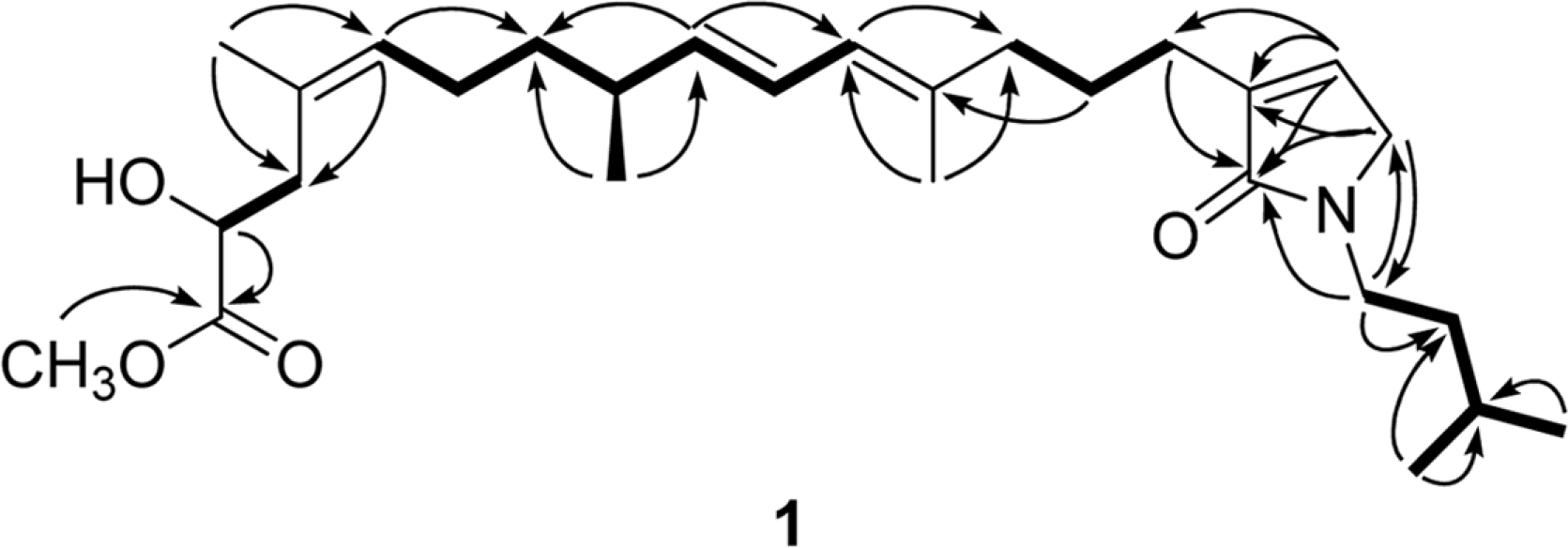
Sarcotragin C (1), a new sesterterpene metabolite was isolated from a Sarcotragus sp. sponge collected from Chuja Island, Korea. On the basis of the combined spectroscopic analyses, the structure of this compound was determined to be a linear norsesterterpene containing a leucine-derived γ-lactam moiety. This compound exhibited moderate cytotoxicity against K562 and A549 cell-lines.
REFERENCES
(1). Blunt J. W., Copp B. R., Keyzers R. A., Munro M. H. G., Prinsep M. R.Nat. Prod. Rep. 2015; 32:116–211.
(2). Liu Y., Zhang S., Jung J. H., Xu T. Z.Naturforsch. C. 2007; 62:473–476.
(3). Shin J., Rho J. R., Seo Y., Lee H. S., Cho K. W., Sim C. J.Tetrahedron Lett. 2001; 42:3005–3007.
(4). Liu Y., Bae B. H., Alam N., Hong J., Sim C. J., Lee C. O., Im K. S., Jung J. H. J.Nat. Prod. 2001; 64:1301–1304.
(5). Liu Y., Hong J., Lee C. O., Im K. S., Kim N. D., Choi J. S., Jung J. H. J.Nat. Prod. 2002; 65:1307–1314.
(6). Liu Y., Mansoor T. A., Hong J., Lee C. O., Sim C. J., Im K. S., Kim N. D., Jung J. H. J.Nat. Prod. 2003; 66:1451–1456.
(7). Barrow C. J., Blunt J. W., Munro M. H. G., Perry N. B. J.Nat. Prod. 1988; 51:275–281.
(8). Barrow C. J., Blunt J. W., Munro M. H. G. J.Nat. Prod. 1989; 52:346–359.
(9). González A. G., Rodríguez M. L., Barrientos A. S. M. J.Nat. Prod. 1983; 46:256–261.
(10). Abed C., Legrave N., Dufies M., Robert G., Guérineau V., Vacelet J., Auberger P., Amade P., Mehiri M.Mar. Drugs. 2011; 9:1210–1219.
(11). He W., Lin X., Xu T., Jung J. H., Yin H., Yang B., Liu Y.Chem. Nat. Compd. 2012; 48:208–210.
(12). Bergquist P. R., Wells R. J.Marine Natural Products: Chemical and Biological Perspectives. Scheuer P. J., editorAcademic Press;New York: 1983. p. 1–50.
(13). Mosmann T. J.Immunol. Methods. 1983; 65:55–63.
(14). Ulukaya E., Ozdikicioglu F., Oral A. Y., Demirci M.Toxicol. in Vitro. 2008; 22:232–239.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download