Abstract
Objective
Ventricular enlargement following head injury is a frequent finding but cases requiring shunt operation are rare. The incidence and developing factors of post-traumatic hydrocephalus (PTH) have been variously reported, but studies for factors influencing outcomes of shunt operation for PTH are rare. The incidence of PTH requiring shunt operation, causing injuries, and factors influencing outcome of shunt operation need to be identified.
Methods
In total, 1,142 patients suffering from traumatic brain injury (TBI) between January 2007 and December 2012 were admitted to our department. Of them, 23 patients underwent shunt operation for diagnosed PTH. In this clinical study, we reviewed retrospectively our TBI database and in the 23 patients, we evaluated outcomes with Glasgow Outcome Score just before the operation, at 14 days, 3 months, and 6 months according to initial Glasgow Coma Scale (GCS) score, interval time between shunt operation and trauma, and lumbar cerebrospinal fluid (CSF) pressure.
Results
The incidence of PTH treated with shunt operation was 2.01%. Subdural hematoma (SDH) was the most common preceding head injury. The outcomes of shunt operation were not related with lumbar CSF pressure or interval time from trauma, but initial GCS score correlated with the outcome.
Conclusion
In present study, 2.01% of TBI patients underwent shunt operation. SDH was the most common preceding injury. Admission GCS score was related to the outcome of shunt operation. However, there were no correlation between the outcome of shunt operation and initial lumbar CSF pressure or interval time of shunt operation after the trauma.
Ventricular enlargement following head injury is a frequent finding but cases that require shunt operation are relatively rare.2) Hydrocephalus following traumatic brain injury (TBI) or post-traumatic hydrocephalus (PTH) is not just a ventricular enlargement but an active and progressive disorder of cerebrospinal fluid (CSF) accumulation in the ventricular system, causing compression of the brain parenchyme.4) It increases the rates of morbidity and mortality in TBI patients, but it is a treatable complication of TBI.11420) Most patients with PTH underwent shunt operation have had favorable outcomes.20) Many studies have reported various incidences and risk factors, under different diagnostic criteria, but few studies have focused on factors influencing outcome of shunt operation for PTH. In this study, we analyzed the incidence, major clinical injuries prior to shunt operation, and factors influencing outcome of shunt operation for patients diagnosed with hydrocephalus following TBI at our institution.
From January 2007 to December 2012, the subjects were the 1,142 consecutive patients suffering from TBI. The main diseases/conditions were skull fracture, cerebral contusion (including diffuse axonal injury) or traumatic intracerebral hematoma (T-ICH), epidural hematoma (EDH), subdural hematoma (SDH), and traumatic subarachnoid hemorrhage (T-SAH). Clinical data were collected retrospectively.
Of them, 23 patients underwent shunt operations. The presenting symptoms or syndrome for considering shunt operation were mental deterioration during improvement, failure to improve (neurologic plateau) for unconscious patients, decline in functions of daily living, headache, gait disturbance, urinary incontinence, confusion, and emotional lability etc. for conscious patients. Before shunt operation, all patients were checked or followed up serially by brain computed tomography (CT), brain magnetic resonance imaging (MRI), radionuclide isotope (RI) cisternography, and serial lumbar drainage (20 to 40 cc per drainage, 1-day to 1-week intervals, more than twice). Indicated brain CT findings were ventricular enlargement with periventricular low density, cortical sulci effacement, and basal cistern obliteration. Brain MRI was used to identify periventricular edema. RI cisternography was assessed for CSF stasis or reflux in the ventricles. They were selected by presenting any symptomatic improvement after serial lumbar drainage.
Shunt operations were ventriculoperitoneal shunt in all 23 patients. Codman-Hakim programmable valves were implanted.
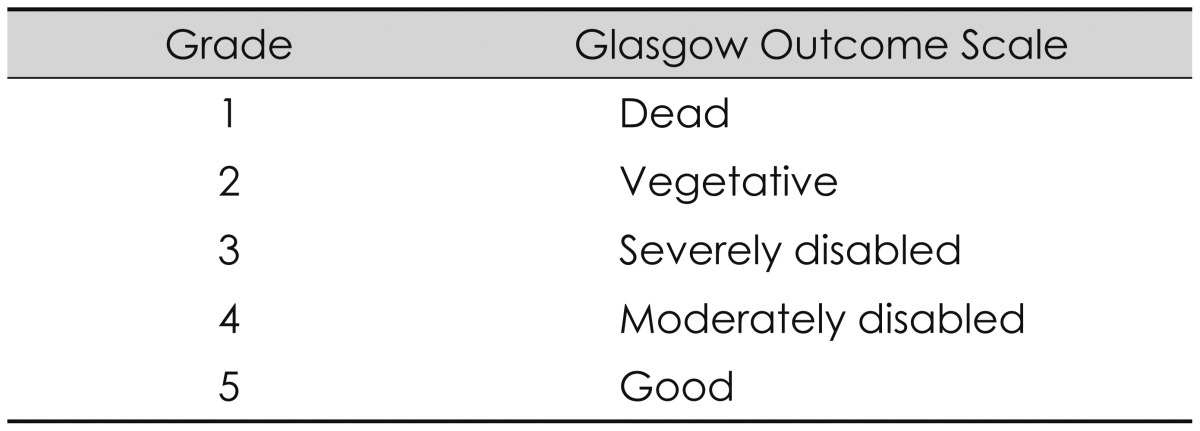
The outcomes of shunt operation were evaluated with the Glasgow Outcome Score (GOS) (Table 1). Evaluation times were just before the shunt operation, and at 14 days, 3 months, and 6 months after the shunt operation. Statistical analyses were made using the χ2 test. p-values <0.05 were considered to indicate statistical significance.
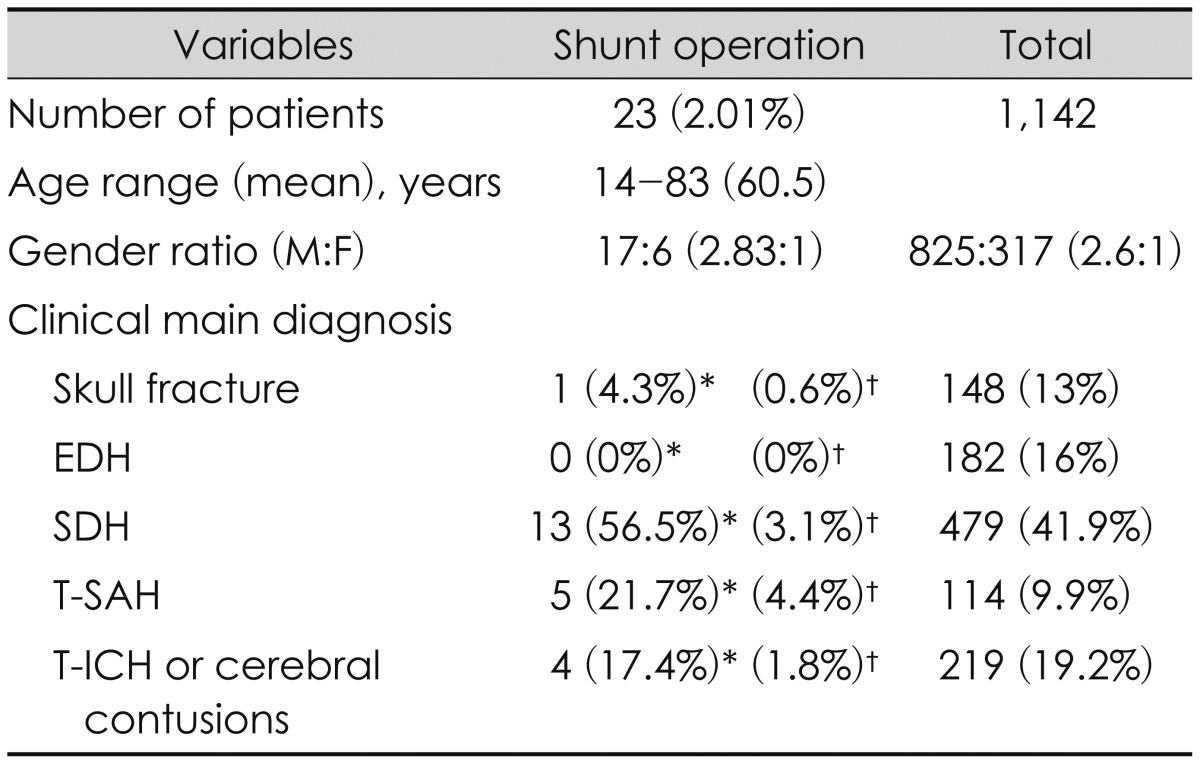
The 23 (2.01%) analyzed subjects were those who underwent shunt operation among 1,142 TBI patients. Their mean age was 60.5 years (range, 14-83 years). There were 17 males and 6 females (2.83:1). Thus, males were about three times more common than females, but this did not differ from the whole TBI patient group (2.6:1). For the main clinical disease, SDH was the most common (56.5%), followed by T-SAH (21.7%), and T-ICH or cerebral contusions (17.4%), in that order (Table 2). Among the 23 PTH patients, 16 had undergone open cranial operation for the removal of hematomas before the shunt operation (Figure 1). Complications occurred in two of the 23 patients. One was ventriculoperitoneal and the other was shunt malfunction.
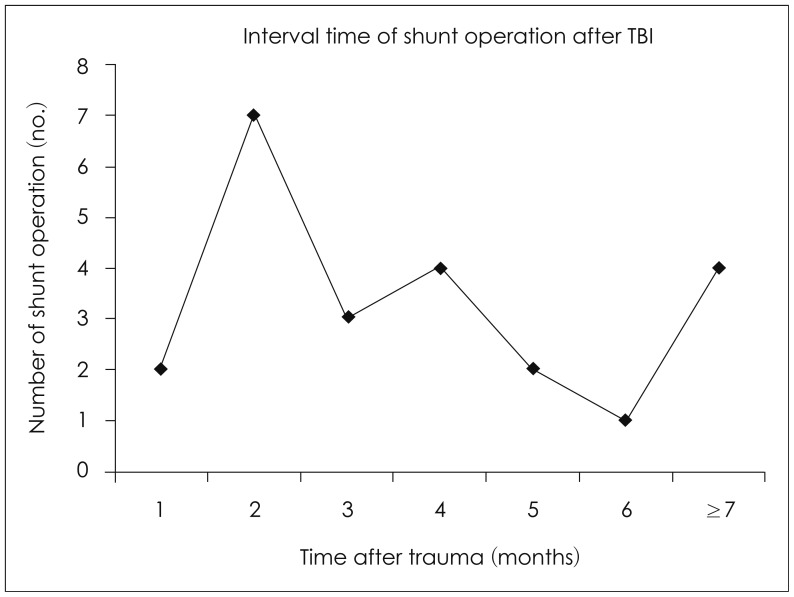
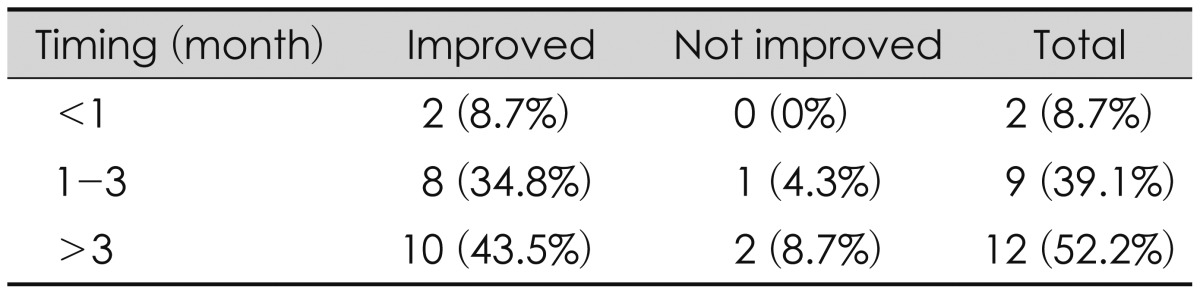
The interval between trauma and shunt operation was analyzed: 2 within 1 month, 7 in a 1-2 months (most common), 3 in 3 months, 4 in 4 months, 2 in 5 months, 1 in 6 months, and 4 after over 6 months. Among them, 1 patient was followed for 1 to 2 years and underwent shunt operation (Figure 2). In the analysis of outcomes related the interval time of shunt operation, 2 of 2 who underwent shunt operation within 1 month improved, 8 of 9 who underwent the operation in 1 to 3 months improved, and 10 of 12 who underwent the operation after 3 months also improved. Thus, it seemed that the interval time between shunt operation and trauma had no effect (p>0.05).
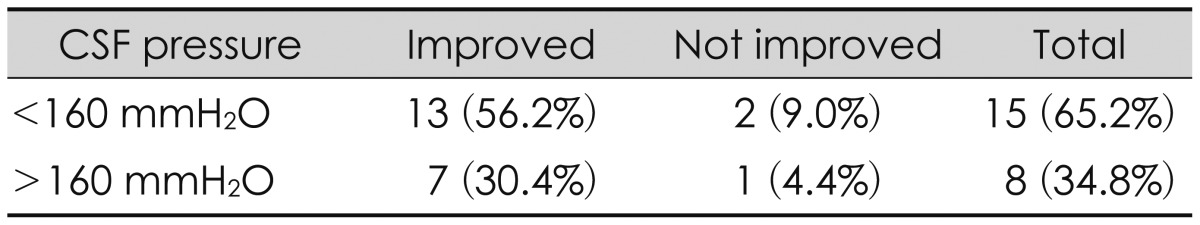
Prior to the shunt operation, we performed lumbar puncture in all patients. Outcomes related to CSF pressure at lumbar spinal tapping prior to shunt operation were analyzed. The group was divided into two: CSF pressure above and below 160 mmH2O.13) Of 15 patients who showed CSF pressures below 160 mmH2O, 13 improved, as did 7 of 8 patients who had CSF pressures above 160 mmH2O (p=0.955). Thus, intracranial pressure (ICP) prior to the shunt operation also seemed to have no significant relationship with prognosis.
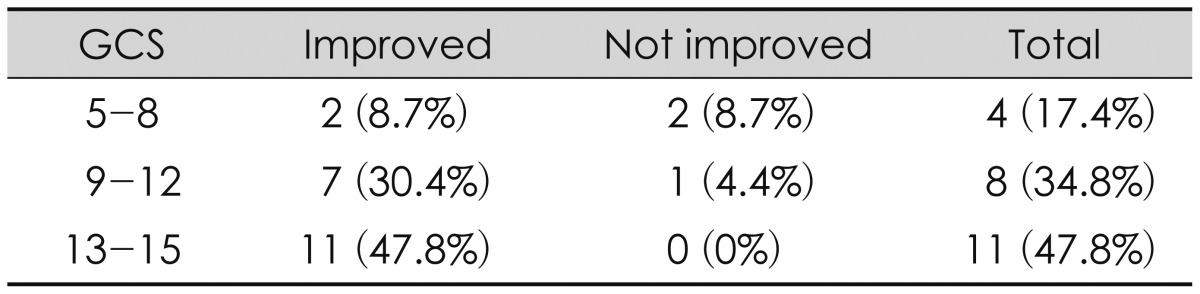
The Glasgow Coma Scale (GCS) score at admission was classified as GCS 5 to 8, 9 to 12, and 13 to 15 groups. Two of 4 (8.7%) in the GCS 5 to 8 group, 7 of 8 (4.4%) in the GCS 9 to 12 group, and all patients in the 13 to 15 group improved (p=0.039, which is statistically significant). Thus, initial GCS score would seem to be related to the outcome of shunt operation.
PTH as a clinicopathologic entity has been recognized since Dandy and Blackfan's report6) in 1964. The incidence rates of symptomatic PTH in the literature vary widely, ranging from 0.7% to 29%.3912) If the CT criterion of ventriculomegaly is used, the reported incidence ranges from 30% to 86%.1018) Gudeman et al.10) reported the incidence of ventricular enlargement after TBI between 1.5% to 29%, when evaluated by CT. But the incidence rate of PTH requiring shunt operation was only 1% to 4%.2)
In the present study, 1,142 patients were admitted to our institution following significant head trauma. Of them, 23 patients underwent ventricular shunt operation. Thus the incidence of PTH requiring shunt operation was 2.01%. Tribl and Oder20) reported that out of 3,426 severe head-injury patients, 48 (1.4%) underwent implantation of ventricular shunt.
Risk factors for PTH are not yet fully identified but data suggested severity of injury, age, duration of coma, and decompressive craniectomy (DC) increased the risk.5) Previous studies suggested that factors such as altered ICP dynamics, mechanical blockage, and inflammation of the arachnoid granulation by postsurgical debris may induce PTH.781721) Waziri et al.21) suggested that DC may play a role in the flattening of the normally dicrotic ICP waveform in patients having the procedure, due to the transmission of the pressure pulse out through the open cranium. The size of DC and repeated operation may promote PTH in severe head trauma patients who undergo DC.4) In the present study, 16 (70%) patients underwent decompressive operation before shunt operation. Two (9%) patients had reoperation before shunt operation.
General risks related to shunt surgery include infection, bleeding, CSF leakage, seizures, and neurological deficits. There is an estimated 3% to 4% risk for intracerebral hemorrhage and an estimated 1% to 2% risk for coma and mortality.14) In contrast, Tribl and Oder20) reported that 15 (31.3%) patients showed postoperative complication. Postoperative complication occurred in 2 (0.9%) patients in our study. This ratio is considered to be quite low versus other reported studies. Reasons for the low incidence of complications may be affected by the small number of patients and short follow-up period.
SAH has been cited as the most important pathology leading to the development of PTH.1314) We found SDH in 13 (56.5%) patients and SAH in 5 (21.7%). Of the 23 patients, 22 (96%) had intradural pathology. For skull fracture patient, it seems that PTH was caused by meningitis during the observation. SDH was the most commonly found issue in initial CT scans in PTH patients who underwent shunt operation. The reason that SDH is the most common preceding injury for shunt operation suspected that SDH is the largest number of intradural lesion in our TBI pool (Table 2). Hematomas on the cerebral convexities may induce inflammation or adhesions to arachnoid granulations. It causes disturbance of CSF absorption to make communicating hydrocephalus. EDH had no effect on PTH (Table 2). The reason seems that EDH is almost extradural lesion. Its intradural effect can be ignored.
One of the predictive parameters for outcome after shunt implantation appeared to be pre-operative clinical status. Patients in better clinical condition generally had a better outcome.1420) In the GCS 13-15 group, 11 (100%) patients showed improved outcomes. However, in the GCS 5 to 8 group, 2 (50%) patients showed improved outcomes. Tribl and Oder20) reported similar results. He investigated the outcome of 48 patients after severe brain injury. His patients in a better pre-operation level of GOS 3 improved in 63.3% of cases, whereas 36.7% did not improve. However, only one-third of patients with GOS 2 before shunt implantation improved within 3 months; two-thirds did not. All current neurosurgical and neurocritical care interventions target the delayed effects of secondary injuries and secondary insults. More severe brain injury occurred more often after the development of PTH. Even though the CSF flow disorder was treated by the shunt surgery, effects of the brain injury are thought to remain. So, higher GCS patients improved after the shunt operation.
In our study, all patients were evaluated by brain CT and RI cisternography. Lumbar CSF pressure was monitored. Serial lumbar drainages were performed in all patients. The initial lumbar CSF pressures were divided two groups, less than 160 mmH2O (15 patients) and higher than 160 mmH2O (8 patients).13) In the less than 160 mmH2O group, 13 (56.2%) patients showed improved outcomes. In the higher than 160 mmH2O group, 7 (30.4%) patients showed improved outcomes. Thus, we found no correlation between the lumbar pressure and postoperative outcome improvement. This result is consistent with the results reported by Kim et al.14) They reported outcomes of 64 PTH patients requiring shunt. Symptoms improved after shunt operation in 46 patients (90%) with normal pressure hydrocephalus. This outcome was better than the improvement seen in only 6 (46.1%) patients who had higher than 180 mmH2O of spinal pressure before the shunt operation.
The timing of treatment also remains controversial.11) PTH can present acute, subacute, and syndrome of normal pressure hydrocephalus. Kishore et al.15) reported that more than 93% of cases of ventriculomegaly presented within 2 weeks post-injury. Marmarou et al.16) suggested that most cases of ventriculomegaly will present by 1 month post-injury. We divided patients into three groups for the interval between trauma and shunt surgery. Most of our patients received shunt operation within 6 months after trauma. In this study, interval time and postoperative improvement showed no correlation. On the other hand, this result showed that most patients were improved after shunt operation, regardless of the operation interval time. Wood et al.22) insisted that patients with clinical symptoms of hydrocephalus for less than 6 months had a better prognosis. However, Sheffler et al.19) presented a case of PTH that improved with a shunting procedure after having clinical symptoms of PTH for 11 months after a closed head injury.
Brain injury patients commonly have other severe injuries (e.g., long bone fracture, intestine organ rupture). For these patients, this result suggests a basis that the shunt operation can be delayed. We often found PTH in patients with head trauma during the follow-up period. In these cases, contrary to assuming a poor prognosis due to the possibility of delayed shunt surgery, we suggest to do shunt operation regardless of the interval time with trauma.
Finally, this study has the limitations of being a retrospective study with a small sample size and lack of natural history of PTH. More long-term follow-up and prospective studies are needed.
In present study, 2.01% of TBI patients underwent shunt operation. SDH was the most common preceding head injury for PTH. Admission GCS score is related to the outcome of shunt operation. However, we found no correlation between the outcome of shunt operation and initial lumbar CSF pressure or the interval time of the shunt operation after the trauma.
References
2. Bret P, Hor F, Huppert J, Lapras C, Fischer G. Treatment of cerebrospinal fluid rhinorrhea by percutaneous lumboperitoneal shunting: review of 15 cases. Neurosurgery. 1985; 16:44–47. PMID: 3974811.

3. Cardoso ER, Galbraith S. Posttraumatic hydrocephalus--a retrospective review. Surg Neurol. 1985; 23:261–264. PMID: 3975808.

4. Choi I, Park HK, Chang JC, Cho SJ, Choi SK, Byun BJ. Clinical factors for the development of posttraumatic hydrocephalus after decompressive craniectomy. J Korean Neurosurg Soc. 2008; 43:227–231. PMID: 19096601.

5. Chuang K, Stroud NL, Zafonte R. Rehabilitation of patients with traumatic brain injury. In : Winn HR, editor. Youmans neurological surgery. ed 6. Phildelphia, PA: Elsevier Saunders;2011. p. 3516–3534. p. e3511–e3513.
6. Dandy WE, Blackfan KD. Internal hydrocephalus. An experimental, clinical and pathological study. J Neurosurg. 1964; 21:588–635.
7. Foltz EL, Ward AA Jr. Communicating hydrocephalus from subarachnoid bleeding. J Neurosurg. 1956; 13:546–566. PMID: 13377208.

8. Foroglou G, Zander E. [Post-traumatic hydrocephalus and measurement of cerebrospinal fluid pressure]. Acta Radiol Diagn (Stockh). 1972; 13:524–530. PMID: 4670773.
9. Groswasser Z, Cohen M, Reider-Groswasser I, Stern MJ. Incidence, CT findings and rehabilitation outcome of patients with communicative hydrocephalus following severe head injury. Brain Inj. 1988; 2:267–272. PMID: 3203174.

10. Gudeman SK, Kishore PR, Becker DP, Lipper MH, Girevendulis AK, Jeffries BF, et al. Computed tomography in the evaluation of incidence and significance of post-traumatic hydrocephalus. Radiology. 1981; 141:397–402. PMID: 6974874.

12. Hawkins TD, Lloyd AD, Fletcher GI, Hanka R. Ventricular size following head injury: a clinico-radiological study. Clin Radiol. 1976; 27:279–289. PMID: 1086181.

13. Khan QU, Wharen RE, Grewal SS, Thomas CS, Deen HG Jr, Reimer R, et al. Overdrainage shunt complications in idiopathic normal-pressure hydrocephalus and lumbar puncture opening pressure. J Neurosurg. 2013; 119:1498–1502. PMID: 23930853.

14. Kim SW, Lee SM, Shin H. Clinical Analysis of Post-traumatic Hydrocephalus. J Korean Neurosurg Soc. 2005; 38:211–214.
15. Kishore PR, Lipper MH, Miller JD, Girevendulis AK, Becker DP, Vines FS. Post-traumatic hydrocephalus in patients with severe head injury. Neuroradiology. 1978; 16:261–265. PMID: 740188.

16. Marmarou A, Foda MA, Bandoh K, Yoshihara M, Yamamoto T, Tsuji O, et al. Posttraumatic ventriculomegaly: hydrocephalus or atrophy? A new approach for diagnosis using CSF dynamics. J Neurosurg. 1996; 85:1026–1035. PMID: 8929491.

17. Pedersen KK, Haase J. Isotope liquorgraphy in the demonstration of communicating obstructive hydrocephalus after severe cranial trauma. Acta Neurol Scand. 1973; 49:10–30. PMID: 4684587.

18. Philippon J, George B, Visot A, Cophignon J. [Post-operative hydrocephalus]. Neurochirurgie. 1976; 22:111–117. PMID: 1012410.
19. Sheffler LR, Ito VY, Philip PA, Sahgal V. Shunting in chronic post-traumatic hydrocephalus: demonstration of neurophysiologic improvement. Arch Phys Med Rehabil. 1994; 75:338–341. PMID: 8129589.

20. Tribl G, Oder W. Outcome after shunt implantation in severe head injury with post-traumatic hydrocephalus. Brain Inj. 2000; 14:345–354. PMID: 10815842.
21. Waziri A, Fusco D, Mayer SA, McKhann GM 2nd, Connolly ES Jr. Postoperative hydrocephalus in patients undergoing decompressive hemicraniectomy for ischemic or hemorrhagic stroke. Neurosurgery. 2007; 61:489–493. discussion 493-494PMID: 17881960.

22. Wood JH, Bartlet D, James AE Jr, Udvarhelyi GB. Normal-pressure hydrocephalus: diagnosis and patient selection for shunt surgery. Neurology. 1974; 24:517–526. PMID: 4499967.

FIGURE 1
A: Seventeen-year-old male was admitted due to a motor cycle accident. Initial brain computed tomography. B: Four months, 20 days after trauma, he underwent a decompressive craniectomy, and a repeated operation for a bilateral subdural hematoma. Cranioplasty was done 40 days after the trauma. Ventricular enlargement developed from about 2.5 months after trauma. Posttraumatic hydrocephalus was confirmed at 140 days after trauma. C: One week after ventriculoperitoneal shunt, the proximal catheter tip is seen in the frontal horn of the right lateral ventricle, which was inserted from the parietal-occipital point under frameless stereotactic guidance. His Glasgow Outcome Score increased from 2 to 3 at 6 months after trauma.
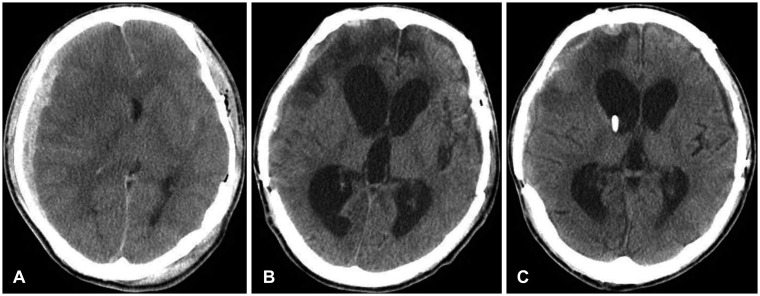
FIGURE 2
Interval time of shunt operation after traumatic brain injury. TBI: traumatic brain injury.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download