Abstract
The major risk factors of hepatocellular carcinoma include hepatitis B or C virus infection and alcohol consumption in Korea which lead to liver cirrhosis development and progression. However, prevalence of non-alcoholic fatty liver disease related hepatocellular carcinoma is rising worldwide and hepatocellular carcinoma cases in patients with non-cirrhotic non-alcoholic steatohepatitis are increasing. A hypoechoic nodule was incidentally detected in a 52-year-old woman, with no evidence of liver cirrhosis or specific hepatocellular carcinoma findings on radiological examination. Non-cirrhotic non-alcoholic steatohepatitis-associated hepatocellular carcinoma was diagnosed based on clinical, laboratory, and histopathological findings of liver biopsy. To our knowledge, this is the first such case report in Korea.
The major risk factors of hepatocellular carcinoma (HCC) include hepatitis B virus (HBV), hepatitis C virus (HCV) infection and alcohol in Korea, but the prevalence of non-alcoholic fatty liver disease (NAFLD)-related HCC is rising worldwide, as 4~22% of HCC cases in Western countries are now attributed to NAFLD [1,2].
Most cases of non-alcoholic steatohepatitis (NASH), an advanced form of NAFLD, do not have poor outcomes, but advancement to liver fibrosis is noted in around 30% of patients and liver cirrhosis develops within 5.6 years in approximately 9% of patients. Among these patients with liver cirrhosis, 4% to 27% eventually develop HCC [3]. Recently, cases of HCC in patients with non-cirrhotic NASH have been reported [4,5].
However, HCC development in patients with NASH who do not first develop liver cirrhosis is rare and has not been previously reported in Korea. Here, we report the first known case of HCC development in a Korean patient with non-cirrhotic NASH.
A hypoechoic nodule was incidentally detected on abdominal ultrasonography in a 52-year-old woman. Right lumpectomy was performed for breast cancer 2 years ago, and the patient was not taking medications. Her family history was unremarkable, and she had a no history of alcohol consumption, smoking, or HBV or HCV infection.
The patient was 155 cm tall, with a body weight of 72.6 kg and body mass index of 30.22 kg/m2. Upon admission, her vital signs were stable and physical examination was unremarkable. The results of laboratory tests were as follows: hemoglobin, 13.6 g/dL; platelet count, 165,000/mm3; albumin, 4.4 g/dL; aspartate aminotransferase (AST), 46 IU/L; alanine aminotransferase (ALT), 63 IU/L; total bilirubin, 0.5 mg/dL; creatinine, 0.7 mg/dL; prothrombin time, 10.5 sec; and alpha fetoprotein, 2.94 ng/mL. The hepatitis examination revealed HBsAg (-), HBsAb (+), HBeAg (-), HBeAb (+), HBcAb (+), HCVAb (-), and HBV DNA <20 IU/mL. The patient had not been previously vaccinated against HBV.
Abdominal ultrasonography showed a fatty liver with a 1.6-cm well-defined nodule (Fig. 1). Computed tomography revealed an enhancing nodule in the arterial and delayed phases. Magnetic resonance imaging showed a nodule with atypical enhancing pattern and low signal intensity in the hepatobiliary phase (Fig. 2). These findings did not suggest liver cirrhosis or specific HCC findings. So, we performed a liver needle biopsy for an accurate diagnosis. A liver needle biopsy demonstrated well-differentiated HCC within NASH background. The patient had a Child-Pugh score of 6, and the indocyanine green test result was 20.1% (range, 0% to 10%). The patient underwent segmentectomy of segment III, as remnant liver function was good. Grossly, a 1.9×1.5 cm sized nodular mass was noted. Microscopically, the mass revealed small, progressed HCC without vascular or lymphatic invasion. Non-tumor parenchyma showed findings consistent with NASH (Fig. 3). Also, HBcAg was not detected in the liver parenchyma. The patient had a successful operation without complications and is being followed-up for 9 months without evidence of recurrence.
To our knowledge, this is the first case of HCC in a patient with non-cirrhotic NASH in Korea. NAFLD-associated HCC was first reported in 1990 [6], and the number of such cases has been increasing. The prevalence of NASH, an advanced form NAFLD, is increasing due to the increasing prevalence of obesity and metabolic syndrome [7]. Therefore, NASH-associated HCC cases are also rising.
The pathogenesis of NASH-related HCC resulting from conditions other than cirrhosis remains unclear, but is considered to be similar to that of liver cirrhosis-associated HCC and is associated with obesity, metabolic syndrome, and diabetes with insulin resistance. Our patient had hepatic fibrosis but not cirrhosis. According to the clinical data of patients with NAFLD-associated HCC from 25 studies published between 1990 and 2010, HCC occurred in the absence of cirrhosis in 40.2% of patients [8]. Therefore, although cirrhotic NASH is a risk factor for HCC development, cirrhosis is not a necessary condition.
Because HBV infection is an etiologic factor for HCC and the prevalence of past infection is higher in Korea than in Western countries, NASH-related HCC cannot be diagnosed unless other causes of HCC, including HBV infection, are ruled out. In this case, the possibility of silent HBV infection could not be completely ruled out because viral serology results (HBsAb, HBeAb, HBcAb) were positive. However, HCC development in cases of occult HBV infection with a negative HBcAg in the liver parenchymal tissue and HBV DNA <20 IU/mL is rare, and NASH was a more important factor in HCC development in this patient.
Risk factors of non-cirrhotic NASH-associated HCC are obesity, metabolic disorder, and old age [9]. In this case, the patient was obese but had no other metabolic disorders. In previous studies, the age at which HCC was diagnosed ranged from 35 to 89 years (median, 67 years); our patient was younger than the median age [8].
Applied to noninvasive panels for the prediction of NASH and significant fibrosis in patients with NAFLD, our patients was NAFLD fibrosis score of 1.38 and BARD score of 1. Thus, it was difficult to determine the risk factors for advanced fibrosis in our patient. Therefore, we performed liver biopsy to rule out other causes of HCC. Histological features of NAFLD-associated HCC, such as micro- and/or macrosteatosis, ballooning, and the presence of Mallory bodies are often observed in noncancerous areas [10]. This patient denied alcohol consumption. We eventually diagnosed non-cirrhotic NASH-associated HCC.
Patients with HCC due to NASH have less severe liver dysfunction at the time of diagnosis and a better overall survival after curative treatment than that with HCV and/or alcoholic liver disease [11]. Therefore, early detection of NASH-associated HCC is important for good prognosis.
In conclusion, simple steatosis should no longer be regarded as a benign condition. NAFLD, especially NASH, is an important risk factor for HCC. Patients with non-cirrhotic NASH could also develop HCC; therefore, regular surveillance for HCC is necessary in such patients and in those with cirrhosis, especially in cases of concurrent metabolic disorders such as obesity or diabetes. Medical treatment is recommended to minimize the risk of HCC development in patients with non-cirrhotic NASH.
Figures and Tables
Fig. 1
Abdominal ultrasonography. It shows a 1.6 cm size well-defined hypoechoic nodule in liver segment III (arrow).
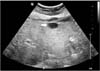
Fig. 2
Liver magnetic resonance imaging (MRI). (A) Liver MRI (arterial phase) shows a well-enhanced and homogenous nodule in the liver segment III (arrow). (B) Liver MRI (hepatobiliary phase) shows a liver nodule with low signal intensity (arrow).
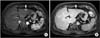
Fig. 3
Histologic findings. (A) Histologic findings of liver reveals diffuse macro- and microvesicular fatty change with moderate ballooning degeneration and moderate lobular activity (H&E, ×100). (B) Histologic findings of liver reveals diffuse periportal fibirosis and occasional bridging fibrosis (Masson's trichrome stain, ×100). (C) Immunohistochemical staining shows negativity at hepatitis B core antigen (×200).

References
1. Ertle J, Dechene A, Sowa JP, Penndorf V, Herzer K, Kaiser G, et al. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011; 128:2436–2443.
2. Malik SM, Gupte PA, de Vera ME, Ahmad J. Liver transplantation in patients with nonalcoholic steatohepatitis-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2009; 7:800–806.
3. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010; 51:1820–1832.
4. Ichikawa T, Yanagi K, Motoyoshi Y, Hamasaki K, Nakao K, Toriyama K, et al. Two cases of non-alcoholic steatohepatitis with development of hepatocellular carcinoma without cirrhosis. J Gastroenterol Hepatol. 2006; 21:1865–1866.
5. Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch Pathol Lab Med. 2008; 132:1761–1766.
6. Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990; 11:74–80.
7. Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007; 17:863–869.
8. Duan XY, Qiao L, Fan JG. Clinical features of nonalcoholic fatty liver disease-associated hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012; 11:18–27.
9. Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013; 10:656–665.
10. Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol. 2007; 128:837–847.
11. Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, et al. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012; 55:1809–1819.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download