Abstract
Nitric oxide (NO) is a small molecule (mol. wt. 30 Da) and oxidative free radical. It is uncharged and can therefore diffuse freely within and between cells across membrane. Such characteristics make it a biologically important messenger in physiologic processes such as neurotransmission and the control of vascular tone. NO is also highly toxic and is known to acts as a mediator of cytotoxicity during host defense.
NO is synthesized by nitric oxide synthase (NOS) through L-arginine/nitric oxide pathway which is a dioxygenation process. NO synthesis involves several participants, three co-substrates, five electrons, five co-factors and two prosthetic groups.
Under normal condition, low levels of NO are synthesized by type I and III NOS for a short period of time and mediates many physiologic processes. Under condition of oxidant stress, high levels of NO are synthesized by type II NOS and inhibits a variety of metabolic processes and can also cause direct damage to DNA. Such interaction result in cytostasis, energy depletion and ultimately cell death. NO has the potential to interact with a variety of intercellular targets producing diverse array of metabolic effects.
It is known that NO is involved in hemodynamic regulation, neurogenic inflammation, re-innervation, management of dentin hypersensitivity on teeth. Under basal condition of pulpal blood flow, NO provides constant vasodilator tone acting against sympathetic vasoconstriction. Substance P, a well known vasodilator, was reported to be mediated partly by NO, while calcitonin-gene related peptide has provided no evidence of its relation with NO.
This review describes the roles of NO in dental pulp in addition to the known general roles of it.
Figures and Tables
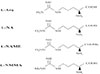
Fig. 1
Schematic representation of the biosynthesis of NO from L-Arginine by the NO synthase system.

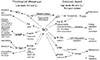
Fig. 2
Synthesis of NO by three isoforms of NOS. (Adapted from Knowles and Moncada, Biochem. J., 1994, 298:249-258.)
References
1. Kim S, Dörscher-Kim J, Kim SK. Contribution of the low compliance environment to the pathophysiology of the pulp. Proceedings of the International Conference on Dentin/Pulp Complex 95 and the International Meeting on Clinical Topics of Dentin/Pulp Complex. 1996. Quintessence Publishing Co., Ltd.;154–157.
2. Knowles RG, Moncada S. Nirtic oxide synthase in mammals. Biochem J. 1994. 298:249–258.
3. Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neuronal role for nitric oxide. Nature. 1990. 347:768–770.

4. Janssens SP, Shimouchi A, Quertermous T, Bloch DB, Bloch KD. Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Bio Chem. 1992. 267:14519–14522.

5. Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992. 89:6348–6352.

6. Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990. 87:682–685.

8. Sessa WC, Hecker M, Mitchell JA, Vane JR. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: L-glutamine inhibits the generation of L-arginine by cultured endothelial cells. Proc Natl Acad Sci U S A. 1990. 87:8607–8611.

9. Kim SK. Role of sympathetic nerve on the control of microcirculation in the feline dental pulp. J Korean Acad Conserv Dent. 1996. 21(1):375–384.
10. Kim SK, Ang L, Hsu YY, Dörscher-Kim J, Kim S. Antagonistic effect of D-myo-inositol-1,2,6-trisphosphate (PP56) on neuropeptide-Y induced vasoconstriction in the feline dental pulp. Arch Oral Biol. 1996. 41(8-9):791–798.

11. Kim SK, Ang L, Hsu YY, Kim S. Effects of sympathetic nerve stimulation, norepinephrine and neuropeptide Y on vasomotor control in the feline dental pulp. Proceedings of the International Conference on Dentin/Pulp Complex 95 and the International Meeting on Clinical Topics of Dentin/Pulp Complex. 1996. Quintessence Publishing Co., Ltd.;232–233.
12. Kim SK, Ang L, Hsu YY, Dörscher-Kim J, Kim S. Characterization of NK1 receptor antagonists in the feline dental pulp. Proceeding of the International Conference on Dentin/Pulp Complex. 2001. 64.
13. Kerezoudis NP, Olgart L, Edwall L. Differential effects of nitric oxide synthesis inhibition on basal blood flow and antidromic vasodilation in rat oral tissues. Eur J Pharmacol. 1993. 14:209–219.

14. Yonehara N, Takamura M, Shigenara Y. Involvement of nitric oxide in re-innervation of rat molar tooth pulp following transection of the inferior alveolar nerve. Brain Res. 1997. 757:31–36.

15. McCormack K, Davies R. The enigma of potassium ion in the management of dentin hypersensitivity: is nitric oxide the evulsive second messenger? Pain. 1996. 68:5–11.

16. Moncada S, Palmer RMJ, Higgs EA. Nitric oxide : physiology, pathology, pathophysiology, and pharmacology. Pharm Rev. 1992. 43(2):109–142.
17. Stamler JS. Redex signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994. 78:931–936.

18. Radomski MW, Palmer RMJ, Moncada S. Characterization of the L-arginine : nitric oxide pathway in human platelet. Br J Pharmacol. 1990. 101:325–328.

19. Radomski MW, Palmer RMJ, Moncada S. An L-arginine/ nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990. 87:5193–5197.

20. Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is nitric oxide synthase. Proc Natl Acad Sci U S A. 1991. 88:2811–2814.
21. Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991. 88:7797–7801.

22. Gabbott PLA, Bacon SJ. Histochemical localization of NADPH-dependant diaphorase(nitric oxide synthase) activity in vascular endothelial cells in the rat brain. Neuroscience. 1993. 57(1):79–95.

23. Law AS, Baumgardner KR, Meller ST, Gebhart GF. Localization and change in NADPH-diaphorase reactivity and nitric oxide synthase immunoreactivity in rat pulp following tooth preparation. J Dent Res. 1999. 78(10):1585–1595.

24. Ekelund U, Mellander S. Role of endothelium derived nitric oxide in the regulation of tones in large-bore arterial resistance vessels, arterioles and veins in cat skeletal muscle. Acta Physiol Scand. 1990. 140:301–309.

25. Glick MR, Gehman JD, Gascho JA. Endothelium-derived nitric oxide reduces baseline venous tone in awake instrumented rats. Am J Physiol. 1993. 265:H47–H51.

26. Lohinai Z, Balla I, Marczis J, Vass Z, Kovach AGB. Evidence for the role of nitric oxide in the circulation of the dental pulp. J Dent Res. 1995. 74(8):1501–1506.

27. Hsu YY. The effect of nitric oxide synthase inhibitor: L-NAME on substance P-induced vasodilation in the feline dental pulp. 1996. University of Pennsylvania;Thesis, Master Sci. in Oral Biol.
28. Hsu YY, Kim SK, Karabucak B, Wong R, Dörscher-Kim J, Kim S. The effect of nitric oxide inhibitor (L-NAME) on substance P induced vasodilation in the feline dental pulp. J Dent Res. 1996. 75(Spec.Iss):357.
29. Olgart L, Kostouros GD, Edwall L. Local actions of acetylcholine on vasomotor regulation in rat incisor pulp. Acta Physiol Scand. 1996. 158:311–316.

30. Berggreen E, Heyeraas KJ. The role of sensory neuropeptides and nitric oxide on pulpal blood flow and tissue pressure in the ferret. J Dent Res. 1999. 78(9):1535–1543.

31. Kim SK, Karabucak B, Welsch H, Simchon S, Kim S. Intracellular mechanism of substance P-induced vosodilatation in bovine dental pulp. J Endod. 2001. 27:231.
32. Kim SK, Karabucak B, Welsch H, Simchon S, Kim S. Vasodilatory function of substance P and L-arginine/nitric oxide pathway. J Dent Res. 2001. 80:552.
33. Walsch H. Neuropeptide-induced vasodilation in bovine dental pulp: the role of calcitonine-gene related peptide in cGMP and cAMP production. 2001. University of Pennsylvania;Thesis, Master Sci. in Oral Biol.
34. Kim SK. Regulatory mechanism of calcitonin-gene related peptide on pulpal blood flow, Project report. 2001. Medical Research Institute, Kyungpook National University Hospital.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download