Abstract
Atopic dermatitis is an well-known skin disease showing inflammatory, chronically relapsing, noncontagious and pruritic symptoms. The aims of this study were to investigate the effects of Liriope platyphylla (LP) on atopic dermatitis of NC/Nga mice. To achieve this, NC/Nga mice were treated with four different conditions including vehicle, phthalic anhydride (PA), PA+5% LP and PA+10% LP, and the changes of immune-related factors were detected after 2 weeks. The pathological phenotypes of atopic dermatitis such as erythema, ear thickness, edema, scab and discharge were significantly decreased in PA+10% LP cotreated groups compare to PA treated group. Also, the weight of lymph node and thymus in immune organs were gradually decreased in LP treated groups, while the weight of spleen was slightly increased in same group. Furthermore, toluidine blue staining analysis, a method used to specifically identify the mast cell, showed that the decrease of master cell infiltration into the dermis were statistically observed in PA+5% LP and PA+10% LP5% cotreated groups. Especially, the decrease of IgE concentration was detected only PA+10% LP cotreated group, although this level was maintained in PA+5% LP cotreated group. Therefore, these results suggested that the water extracts of LP may contribute the relieve of atopic dermatitis symptoms and be considered as an excellent candidate for a atopic dermatitis-therapeutic drug.
References
Bae, E.A., Han, M.J., Shin, Y.W. and Kim, D.H. (. 2006. ). Inhibitory effects of Korean red ginseng and its genuine constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive cutaneous anaphylaxis reaction and contact dermatitis models. Biol. Pharm. Bull. 29(9):1862–1867.
Ban, M. and Hettich, D. (. 2005. ). Effect of Th2 cytokine antagonist treatments on chemical-induced allergic response in mice. J. Appl. Toxicol. 25:239–247.
Boehm, T. and Bleul, C.C. (. 2007. ). The evolutionary history of lymphoid organs. Nat. Immunol. 8(2):131–135.
Choi, M.J., Lee, Y.M., Jin, B.S. and Kim, B.H. (. 2010. ). Inhibitory effect of Luteolin liposome solution by animal model for atopic dermatitis in NC/Nga mice. Lab. Anim. Res. 26(1):47–53.
Choi, S.B., Wha, J.D. and Park, S. (. 2004. ). The insulin sensitizing effect of homoisoflavone-enriched fraction in Liriope platyphylla Wang et Tang via PI3-kinase pathway. Life Sci. 75:2653–2664.
Dearman, R.J. and Kimber, I. (. 1992. ). Divergent immune responses to respiratory and contact chemical allergens: antibody elicited by phthalic anhydride and oxazolone. Clin. Exp. Allergy. 22:241–250.
Dubois, G.R., BruijnzeeI-Koomen, C.A.F.M. and Bruijnzeel, P.L.B. (. 1994. ). IL-4-induced migration of eosinophils in allergic inflammation. Ann. N. Y. Acad. Sci. 725:268–273.
Hur, J., Lee, P., Kim, J., Kim, A.J., Kim, H. and Kim, S.Y. (. 2004. ). Induction of nerve growth factor by butanol fraction of Liriope platyphylla in C6 and primary astrocyte cells. Biol. Pharm. Bull. 27:1257–1260.
Hur, J., Lee, P., Moon, E., Kang, I., Kim, S.H., Oh, M.S. and Kim, S.Y. (. 2009. ). Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. Eur. J. Pharmacol. 620:9–15.
Incorvaia, C., Frati, F., Verna, N., D'Alo, S., Motolese, A. and Pucci, S. (. 2008. ). Allergy and the skin. Clin. Exp. Immunol. 153:27–29.
Jeong, S., Chae, K., Jung, Y.S., Rho, Y.H., Lee, J., Ha, J., Yoon, K.H., Kim, G.C., Oh, K.S., Shin, S.S. and Yoon, M. (. 2008. ). The Korean traditional medicine Gyeongshingangjeehwan inhibits obesity through the regulation of leptin and PPARalpha action in OLETF rats. J. Ethnopharmacol. 119:245–251.
Katon, W., Russo, J., Lin, E.H.B., Heckbert, S.R., Karter, A.J., Williams, L.H., Ciechanowski, P., Ludman, E. and Von Korff, M. (. 2009. ). Diabetes and poor disease control: is comorbid depression associated with poor medication adherence or lack of treatment intensification? Psychosom. Med. 71:965–972.
Kay, A.B. (. 2001. ). Allergy and allergic diseases. N. Engl. J. Med. 344:30–37.
Kim, H.J., Kim, J., Kim, S.J., Lee, S.H., Park, Y.S., Park, B.K., Kim, B.S., Kim, S.K., Cho, S.D., Jung, J.W., Nam, J.S., Choi, C.S. and Jung, J.Y. (. 2010. ). Anti-inflammatory effect of Quercetin on Picryl Chloride-induced contact dermatitis in BALB/c mice. Lab. Anim. Res. 26(1):7–13.
Kim, S.D., Ku, Y.S., Lee, I.Z., Kim, I.D. and Youn, K.S. (. 2001. ). General components and sensory evaluation of hot water extract from Liriopis Tuber. J. Korean Soc. Food Sci. Nutr. 30(1):20–24.
Kim, Y.H., Han, J.K. and Kim, Y.H. (. 2008a. ). Effect of Kami-kanghwalsan (KKHS) on atopic dermatitis-like skin lesions induced in NC/Nga mouse by mite antigen stimulation. J. Korean Oriental Pediatrics. 22(1):69–93.
Kim, Y.H., Lee, S.Y. and Kim, W.I. (. 2008b. ). Antiallergic effects of Cheonmaectang in RBL-2H3 cell. J. Korean Oriental Pediatrics. 22(1):95–102.
Kiwamoto, T., Ishii, Y., Morishima, Y., Yoh, K. and Maeda, A. (. 2006. ). Transcription factors T-bet and GATA-3 regulate development of airway remodeling. Am. J. Respir. Crit. Care Med. 174:142–151.
Krentz, A.J., Patel, M.B. and Bailey, C.J. (. 2008. ). New drugs for type 2 diabetes mellitus: what is their place in therapy? Drugs. 68:2131–2162.
Lee, Y.C., Lee, J.C., Seo, Y.B. and Kook, Y.B. (. 2005. ). Liriopis tuber inhibit OVA-induced airway inflammation and bronchial hyperresponsiveness in murine model of asthma. J. Ethnopharmacol. 101(1–3):144–152.
Mebius, R.E. and Kraal, G. (. 2005. ). Structure and function of the spleen. Nat. Rev. Immunol. 5(8):606–16.
Pabst, R. (. 2007. ). Plasticity and heterogeneity of lymphoid organs. What are the criteria to call a lymphoid organ primary, secondary or tertiary? Immunol. Lett. 112(1):1–8.
Swirski, F.K., Nahrendorf, M., Etzrodt, M., Wildgruber, M., Cortez-Retamozo, V., Panizzi, P., Figueiredo, J.L., Kohler, R.H., Chudnovskiy, A., Waterman, P., Aikawa, E., Mempel, T.R., Libby, P., Weissleder, R. and Pittet, M.J. (. 2009. ). Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 325:612–616.
Umetsu, D.T. and Dekruyff, R.H. (. 2006. ). The regulation of allergy and asthma. Immunol. Rev. 212:238–255.
Figure 1.
Effects of Liriope platyphylla (LP) water extracts on the ear pathological phenotypes, the body weight and the ear thickness. Phthalic anhydride (PA) solution was repeatedly applied to the dorsum of the ear and back skin of NC/Nga mice. After 2 weeks, the difference in the skin irritation between PA treated group and PA+LP cotreated group was determined based on the change of ear pathological phenotype (A), the body weight (B) and the ear thickness (C) using the procedure described in the Materials and Methods. Data shown are the means±SD (n=5). ∗P<0.05 is the significance level compared to the vehicle treated group. ∗∗P<0.05 is the significance level compared to the PA treated group.
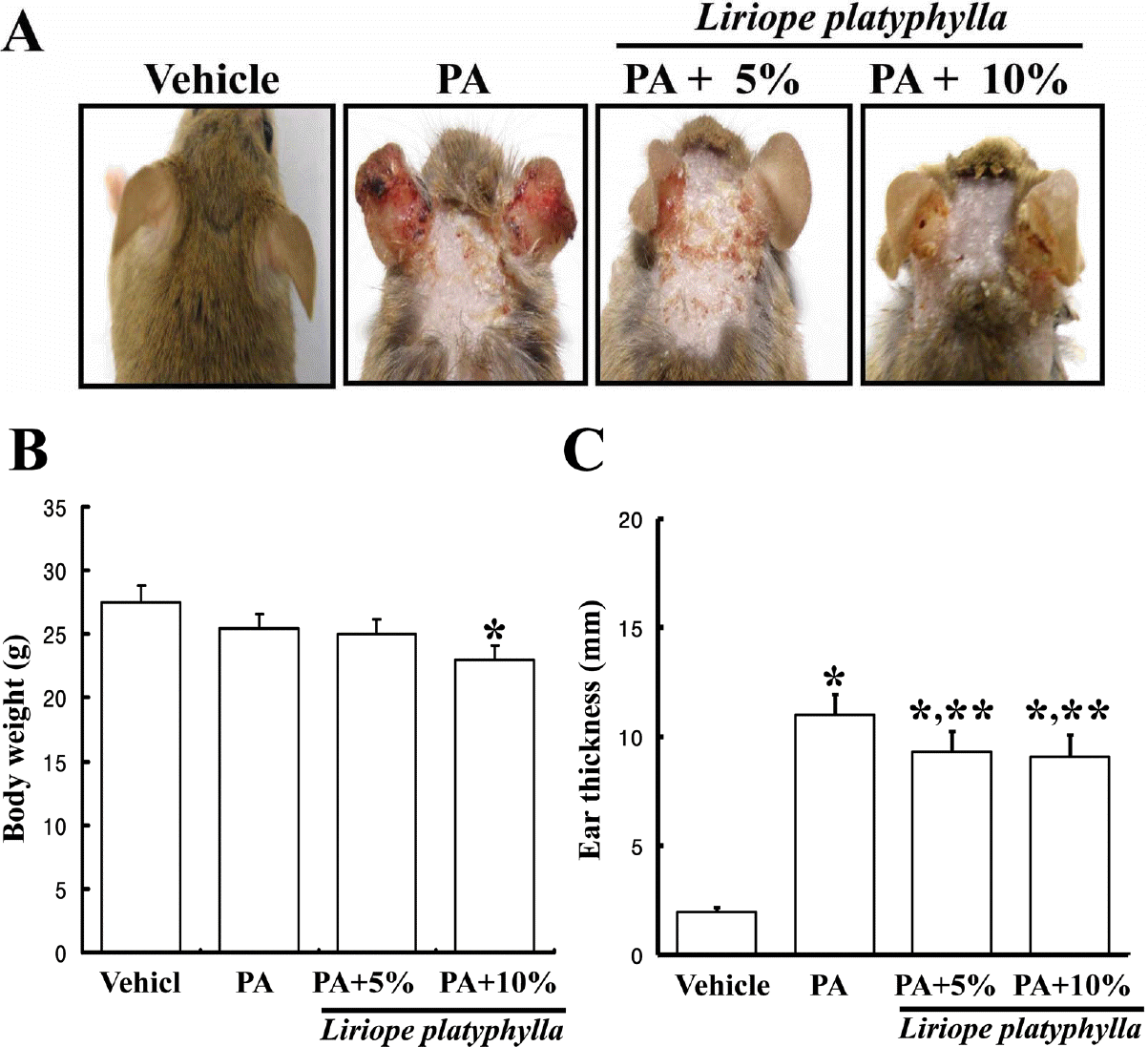
Figure 2.
Effects of Liriope platyphylla (LP) water extracts on weight of three immune organs. At fourteenth days after LP extracts treatment, all of the animals were immediately sacrificed using CO2 gas in order to prepare the immune organs. Lymph nodes, spleens and thymus were collected from animals of all groups. And then, their weight were measured using the chemical balances. Data shown are the means±SD (n=5). ∗P<0.05 is the significance level compared to the vehicle treated group. ∗∗P<0.05 is the significance level compared to the phthalic anhydride (PA) treated group.
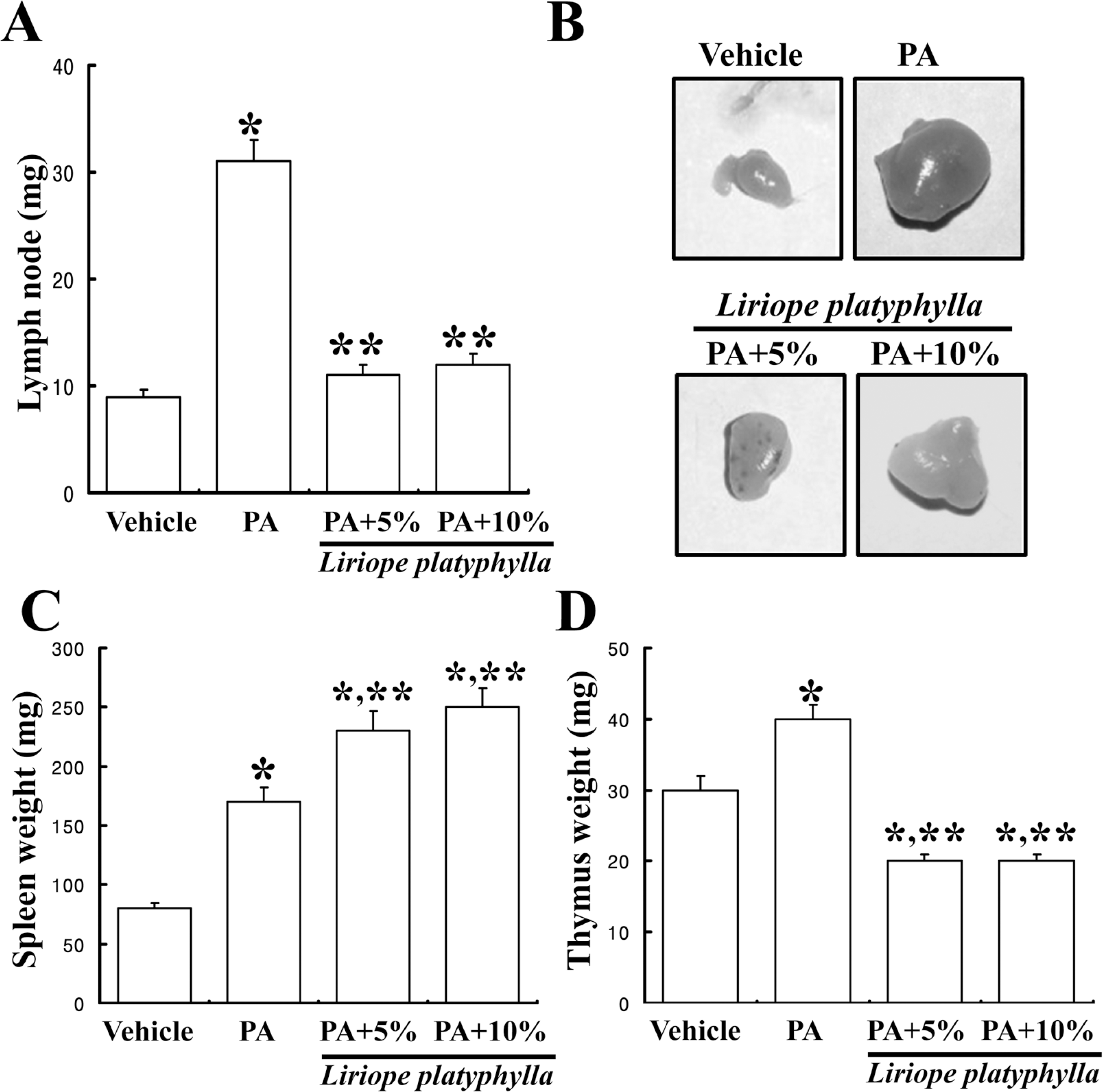
Figure 3.
Effects of Liriope platyphylla (LP) water extracts on the pathological change of ear. The slide sections of ear tissue were stained with hematoxylin & eosin and observed at the original magnification ×40 or ×200. In addition, the arrows indicated the width of ear skin including epidermis, dermis and subcutaneous layer.
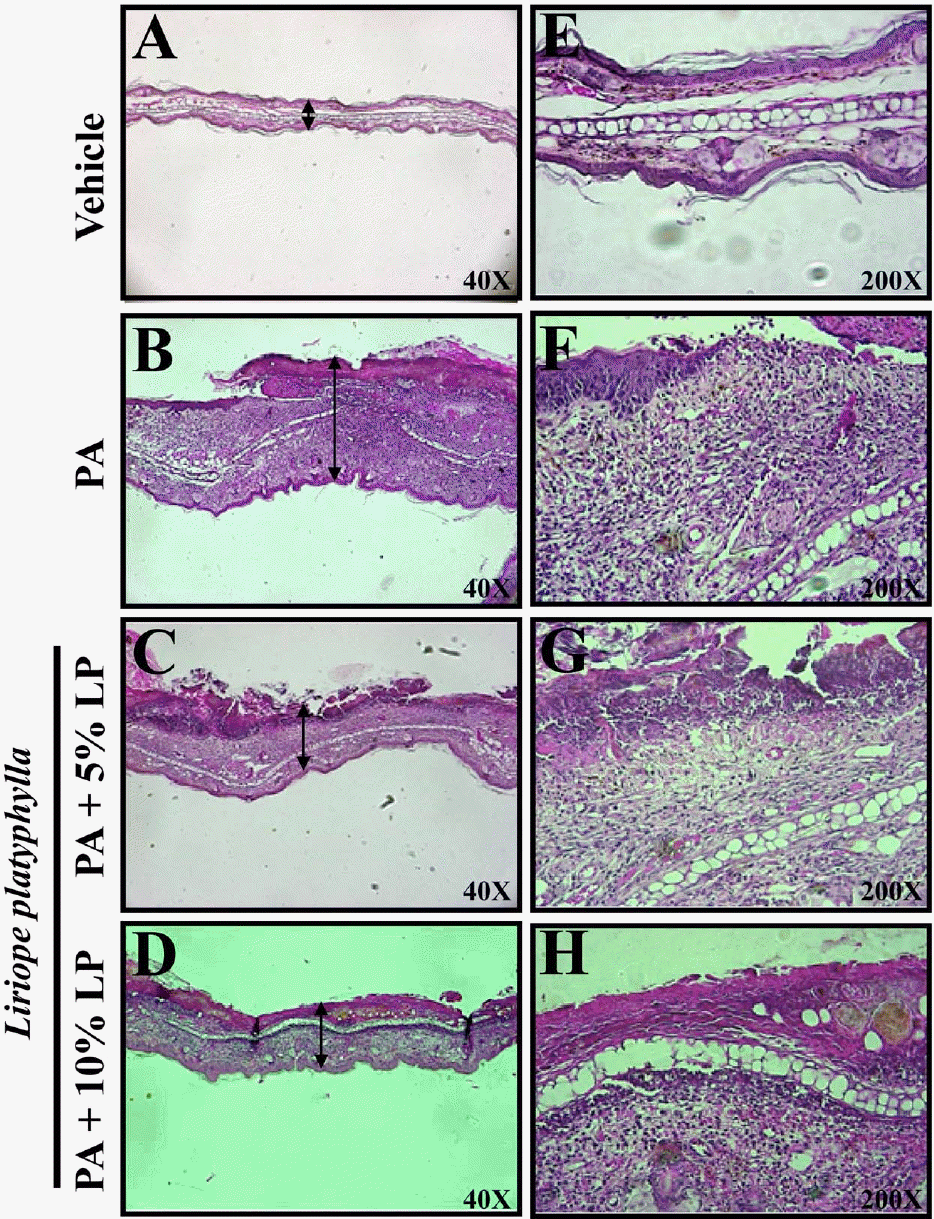
Figure 4.
Effects of Liriope platyphylla water extracts on the mast cell infiltration. (A) The slide sections of ear tissue were stained with toluidine blue and observed at 400x magnification. Mast cells were stained with purple color in the dermis of ear tissue. (B) In each slide, five fields were randomly chosen and the number of mast cells was counted under a light microscope. The values are mean±SD. ∗P<0.05 is the significance level compared to the vehicle treated group. ∗∗P<0.05 is the significance level compared to the phthalic anhydride (PA) treated group.
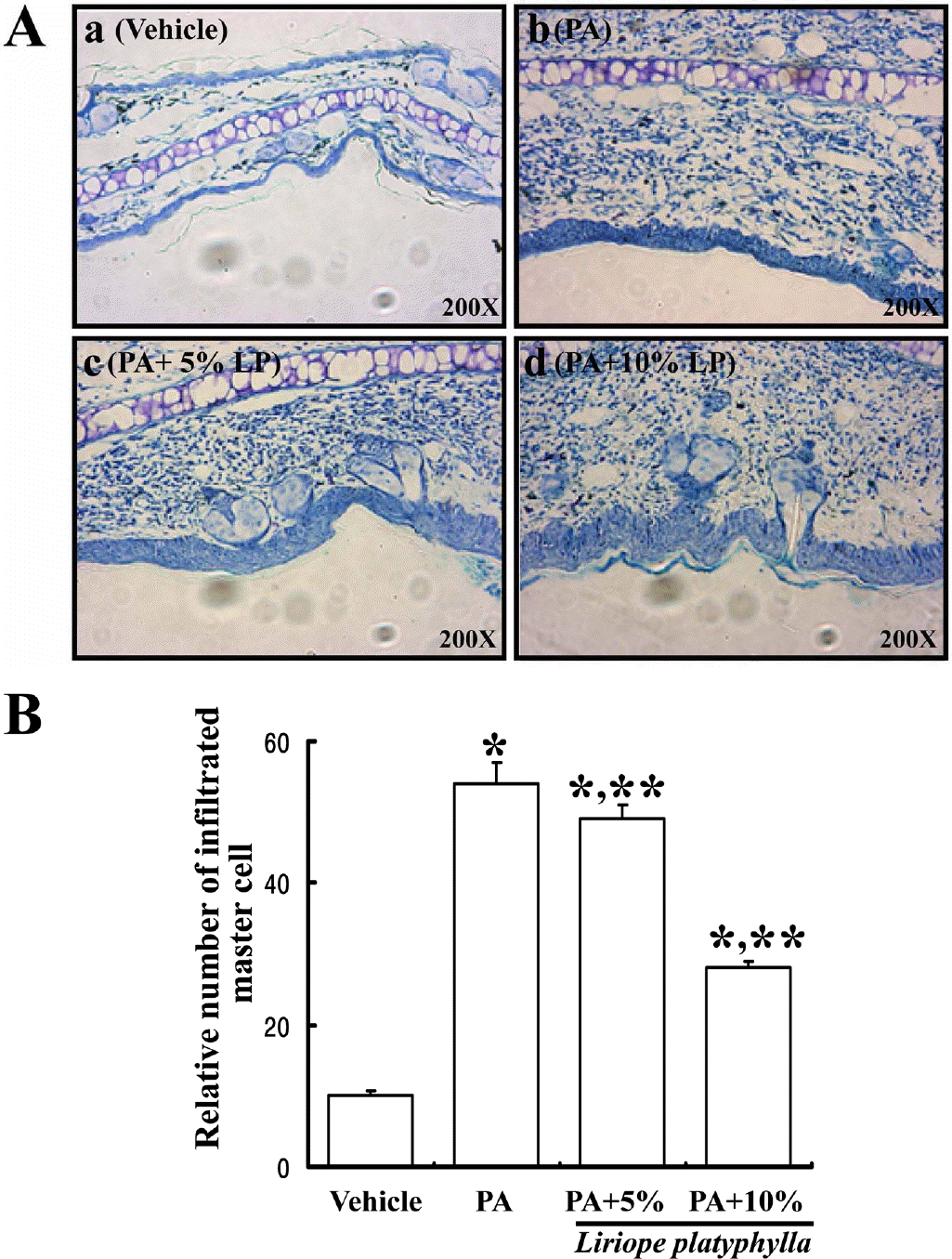
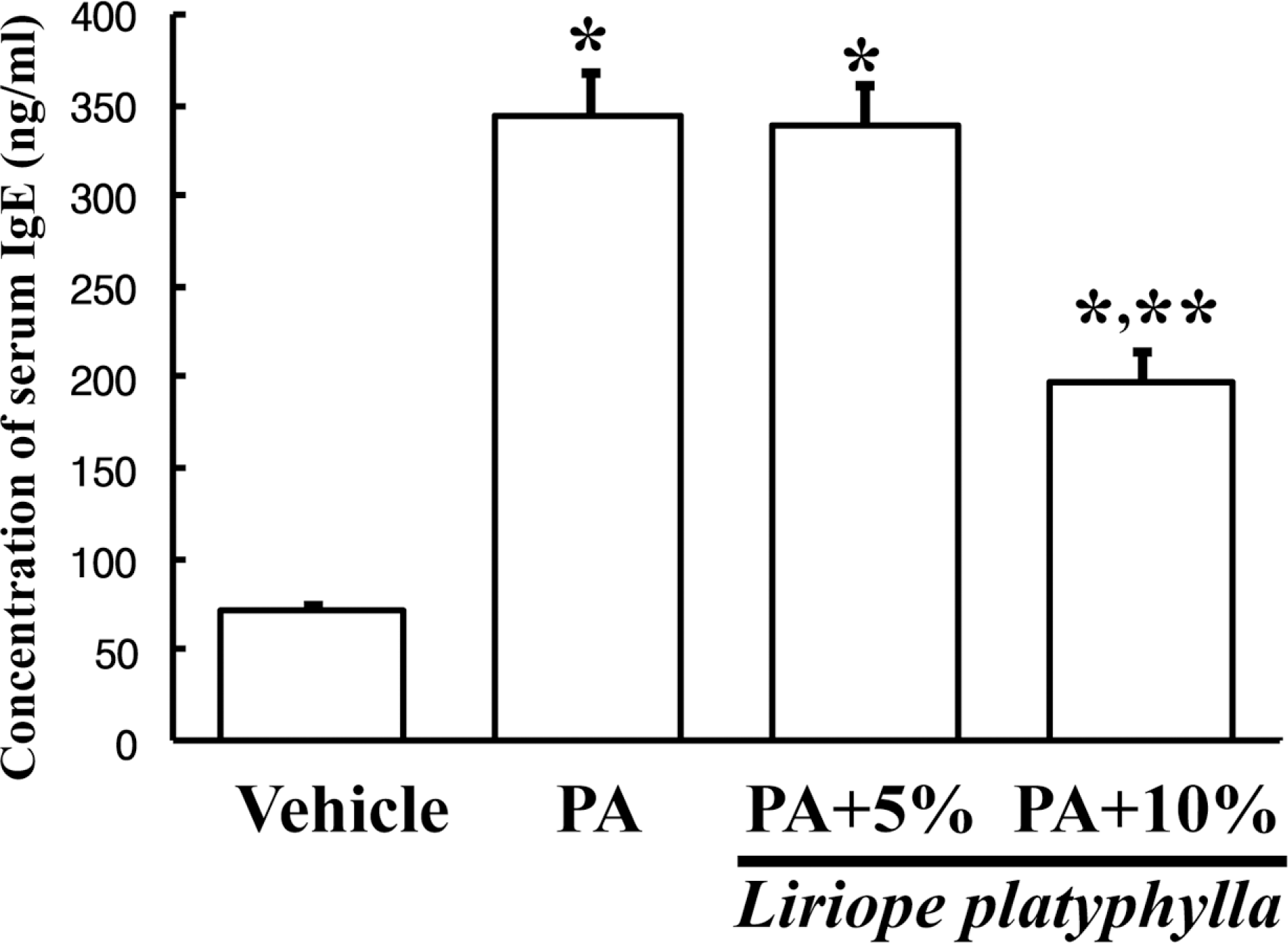
Figure 5.
Effects of Liriope platyphylla (LP) water extracts on the serum IgE concentration. After PA and different concentration of LP cotreatment, the mice were sacrificed under anesthesia. The serum was prepared from blood samples collected from the abdominal vein of the mice. The IgE concentration in serum was quantified by an enzyme-linked immunosorbent assay. Data shown are the means±SD (n=5). ∗P<0.05 is the significance level compared to the vehicle treated group. ∗∗P<0.05 is the significance level compared to the phthalic anhydride (PA) treated group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download