Abstract
Objectives
The objective of this study was to evaluate the efficacy of Unicenta (UNCNT) and Melsmon in women with the menopausal symptoms, by analysing the changes in the Kupperman index (primary endpoint), and the hormonal change (secondary endpoint).
Methods
This is a randomized, multi-Center, double-Blind, parallel, non-inferiority clinical study of four different tertiary medical centers. We began the participant recruitment in September 2011, with 218 patients applied over 7 months. All participants had the last visit in April 2012.
Results
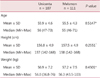
The Unicenta injection was not inferior to that of Melsmon as measured by the Kupperman index following the injection in both the intent-to-treat and the per-protocol populations (P = 0.63, P = 0.85, respectively). Side effects occurred in 14.0% of the cases (15 patients/18 cases) in the case group, and in 12.6% (14 patients/15 cases) in the control group (P value=0.7599). None were reported to be associated with the medication. The laboratory results and the vital signs showed no statistically significant risk for safety.
Figures and Tables
Table 3
Comparison of the change in the Kupperman index from baseline to visit 8 according to the intent-to-treat, per-protocol population

References
1. Lee BI, Koh SK, Hwang SO, Park JH, Kim CW. Comparative study on treatment of postmenopausal symptoms with black cohosh root extract and hormone replacement therapy. Korean J Obstet Gynecol. 2002. 45:1330–1335.
2. Chung DJ, Kim HY, Park KH, Jeong KA, Lee SK, Lee YI, et al. Black cohosh and St. John's wort (GYNO-Plus(R)) for climacteric symptoms. Yonsei Med J. 2007. 48:289–294.
3. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992. 45:769–773.
4. Cohen J. A power primer. Psychol Bull. 1992. 112:155–159.
5. Kim ES, Lee TY, Lee DB, Lee SG, Park MC, Hwang IT. Effects of alcohol drinking on bone mineral density and serum estradiol level in postmenopausal women. J Korean Soc Menopause. 2001. 7:1–22.
6. Park JW, Hur EJ, Lee WK. Effects of an extract from cimicifug a racemosa in menopausal women. Korean J Obstet Gynecol. 2002. 45:117–121.
7. Park HM, Kang BM, Kim JG, Yoon BK, Lee BI, Cho SH, et al. The effect of black cohosh with St. John's wort (Feramin-Q(R)) on climacteric symptoms: multicenter randomized double-blind placebo-controlled trial. Korean J Obstet Gynecol. 2005. 48:2403–2413.
8. Lee DS, Park YS, Kim SY, Cho BY, Lee HK, Koh CS, et al. Clinical effieacy of estradiol patch treatment in the management of postmenopausal syndrome. J Korean Soc Endocrinol. 1990. 5:131–140.
9. Yoon BK, Kim JG, Lee JY, Chang YS, Park YS, Lee DS, et al. Pharmacokinetics and acceptability of estradiol patch treatment in the management of postmenopausal syndrome. J Korean Soc Endocrinol. 1989. 4:203–208.
10. Won HJ, Lee BS, Lee SK, Choi Y, Yoon S, Park KH, et al. The effect of isoflavone on postmenopausal symptoms and hormonal changes in postmenopausal women. J Korean Soc Menopause. 2001. 7:54–63.
11. Chakraborty PD, De D, Bandyopadhyay S, Bhattacharyya D. Human aqueous placental extract as a wound healer. J Wound Care. 2009. 18:462464–467.
12. Nagase M. Clinical assessment of injecting human placental extract into acupuncture points: a case series. East Med. 2005. 21:19–23.
13. Ueno M. Combination treatment of melasma with placenta extract injection. J Jpn Soc Aesthet Surg. 2002. 39:4–8.
14. Hong JW, Lee WJ, Hahn SB, Kim BJ, Lew DH. The effect of human placenta extract in a wound healing model. Ann Plast Surg. 2010. 65:96–100.
15. Nagase M. Placental therapy. East Med. 2005. 20:31–38.
16. Oh EJ, Kim TK, Shin JH, Choi JH, Chung HY. Biologic filler using human fibroblasts and placenta extracts. J Craniofac Surg. 2011. 22:1557–1560.
17. Park SY, Phark S, Lee M, Lim JY, Sul D. Anti-oxidative and anti-inflammatory activities of placental extracts in benzo[a]pyrene-exposed rats. Placenta. 2010. 31:873–879.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download