Abstract
Purpose
We analyzed the clinical outcome of osteosarcoma developed in distal radius and the effect of delayed treatment on prognosis.
Materials and Methods
Twelve patients with distal radius osteosarcoma were analysed. We categorized patients into two groups of standard treatment or non-standard treatment. The patients of standard treatment group are all stage IIB and non-standard treatment group includes five stage IIB and one stage III.
Results
Five-year overall survival and disease-free survival rates of standard treatment group were 100% and 83%. Five-year overall survival rate of non-standard treatment group was 44%. Between two group, there are differences in age, tumor size, surgery type, symptom duration.
Conclusion
Distal radius osteosarcoma have good prognosis than other extremity osteosarcoma. Survival rate of non-standard treatment group were lower than standard treatment group. Although the prognosis of non standard treatment group is poorer, the duration till death was longer than that of other sites with similar condition. Further multi-institutional study should be needed.
REFERENCES
1. Bacci G, Forni C, Longhi A, et al. Local recurrence and local control of non-metastatic osteosarcoma of the extremities: a 27-year experience in a single institution. J Surg Oncol. 2007; 96:118–23.

2. Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing's sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007; 459:40–7.
3. Le Deley MC, Guinebretière JM, Gentet JC, et al. Société Française d'Oncologie Pédiatrique (SFOP). SFOP OS94: a randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. Eur J Cancer. 2007; 43:752–61.
4. Lewis IJ, Nooij MA, Whelan J, et al. MRC BO06 and EORTC 80931 collaborators; European Osteosarcoma Intergroup. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007; 99:112–28.
5. Petrilli AS, de Camargo B, Filho VO, et al. Brazilian Osteosarcoma Treatment Group studies III and IV. Results of the Brazilian Osteosarcoma Treatment Group studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006; 24:1161–8.
6. Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006; 106:1154–61.
7. Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002; 20:776–90.

8. Bieling P, Rehan N, Winkler P, et al. Tumor size and prognosis in aggressively treated osteosarcoma. J Clin Oncol. 1996; 14:848–58.

9. Carsi B, Rock MG. Primary osteosarcoma in adults older than 40 years. Clin Orthop Relat Res. 2002; 397:53–61.

10. Kim MS, Lee SY, Lee TR, et al. Prognostic nomogram for predicting the 5-year probability of developing metastasis after neoadjuvant chemotherapy and definitive surgery for AJCC stage II extremity osteosarcoma. Ann Oncol. 2009; 20:955–60.

11. Kim MS, Lee SY, Cho WH, et al. An examination of the efficacy of the 8 cm maximal tumor diameter cutoff for the subdivision of AJCC stage II osteosarcoma patients. J Surg Oncol. 2008; 98:427–31.

12. Kim MS, Lee SY, Cho WH, et al. Initial tumor size predicts histologic response and survival in localized osteosarcoma patients. J Surg Oncol. 2008; 97:456–61.

13. Lee JA, Kim MS, Kim DH, et al. Risk stratification based on the clinical factors at diagnosis is closely related to the survival of localized osteosarcoma. Pediatr Blood Cancer. 2009; 52:340–5.

14. Pakos EE, Nearchoua AD, Grimer RJ, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer. 2009; 45:2367–75.

15. Bramer JA, van Linge JH, Grimer RJ, Scholten RJ. Prognostic factors in localized extremity osteosarcoma: a systematic review. Eur J Surg Oncol. 2009; 35:1030–6.

16. Cho WH, Song WK, Jeon DG, et al. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Ann Surg Oncol. 2010; 17:702–8.

17. Daecke W, Bielack S, Martini AK, et al. Osteosarcoma of the hand and forearm: experience of the cooperative osteosarcoma study group. Ann Surg Oncol. 2005; 12:322–31.

18. Gobel V, Jurgens H, Etspuler G, et al. Prognostic significance of tumor volume in localized Ewing's sarcoma of bone in children and adolescents. J Cancer Res Clin Oncol. 1987; 113:187–91.
19. Kim MS, Lee SY, Cho WH, et al. Tumor necrosis rate adjusted by tumor volume change is a better predictor of survival of localized osteosarcoma patients. Ann Surg Oncol. 2008; 15:906–14.

20. Kim MS, Cho WH, Song WS, Lee SY, Jeon DG. Time dependency of prognostic factors in patients with stage II osteosarcomas. Clin Orthop Relat Res. 2007; 463:157–65.

21. Rosen G, Marcove RC, Huvos AG, et al. Primary osteogenic sarcoma: eight-year experience with adjuvant chemotherapy. J Cancer Res Clin Oncol. 1983; 106(Suppl):55–67.

22. Bentzen SM, Poulsen HS, Kaae S, et al. Prognostic factors in osteosarcomas. A regression analysis. Cancer. 1988; 62:194–202.

23. Goorin AM, Perez-Atayde A, Gebhardt M, et al. Weekly high-dose methotrexate and doxorubicin for osteosarcoma: The Dana-Farber Cancer Institute/the Children's Hospital-study III. J Clin Oncol. 1987; 5:1178–84.

24. Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of non-metastatic osteosarcoma of the extremities with preoperative and postoperative chemotherapy: a report of the Children's Cancer Group. J Clin Oncol. 1997; 15:79–84.
Figure 1.
(A) Initial plain radiograph of 6-year-old patient (Case 1) shows osteoblastic lesion with periosteal reaction on distal radius. (B) Wide excision and wrist arthrodesis was done with pasteurized bone and Ender nail.
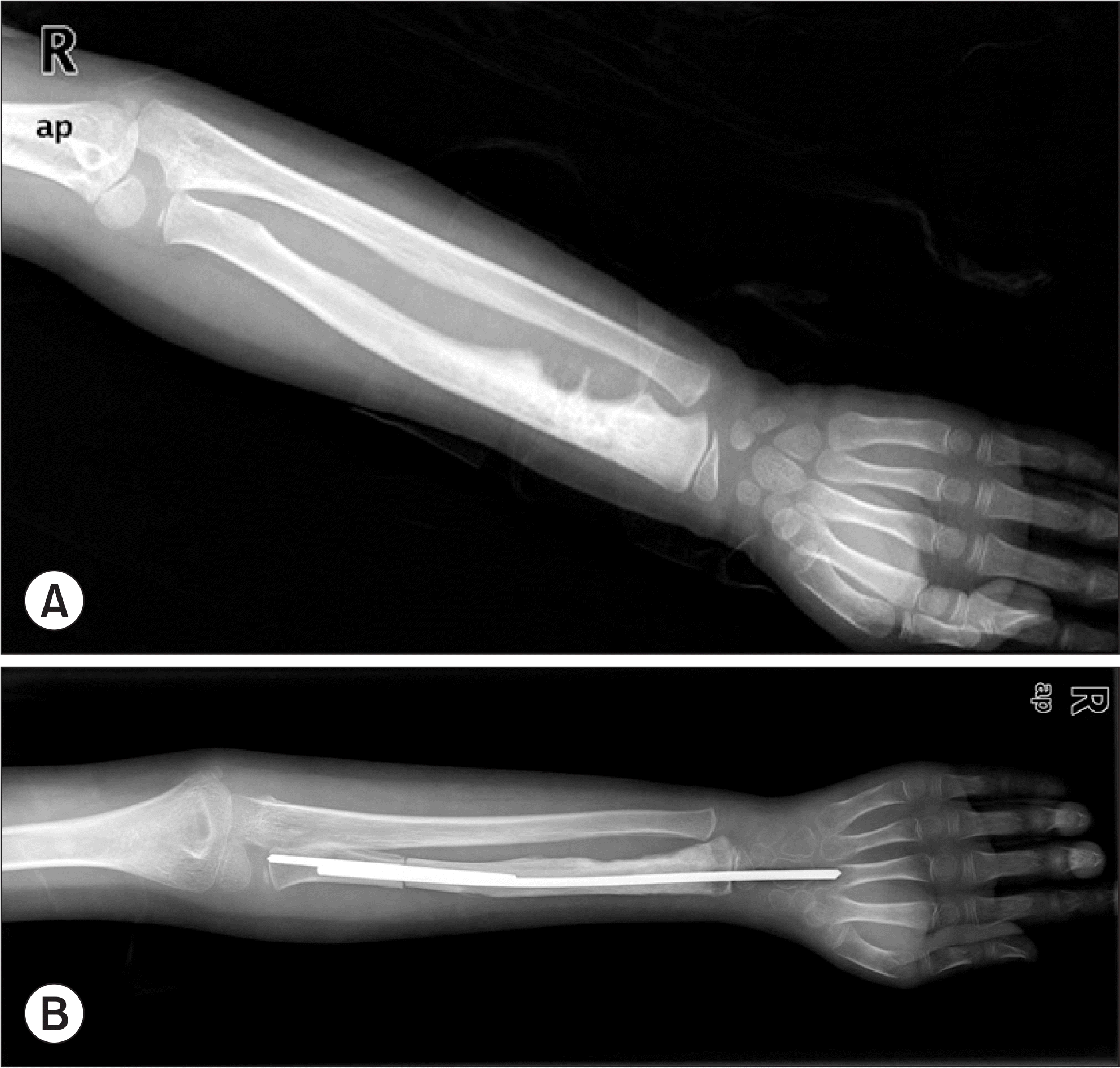
Figure 2.
Radiograph of 53-year-old female taken 15 months after curettage at other hospital shows huge bulging mass with bone destruction. She was treated with amputation and adjuvant chemotherapy, but died with metastasis 4 months after operation.
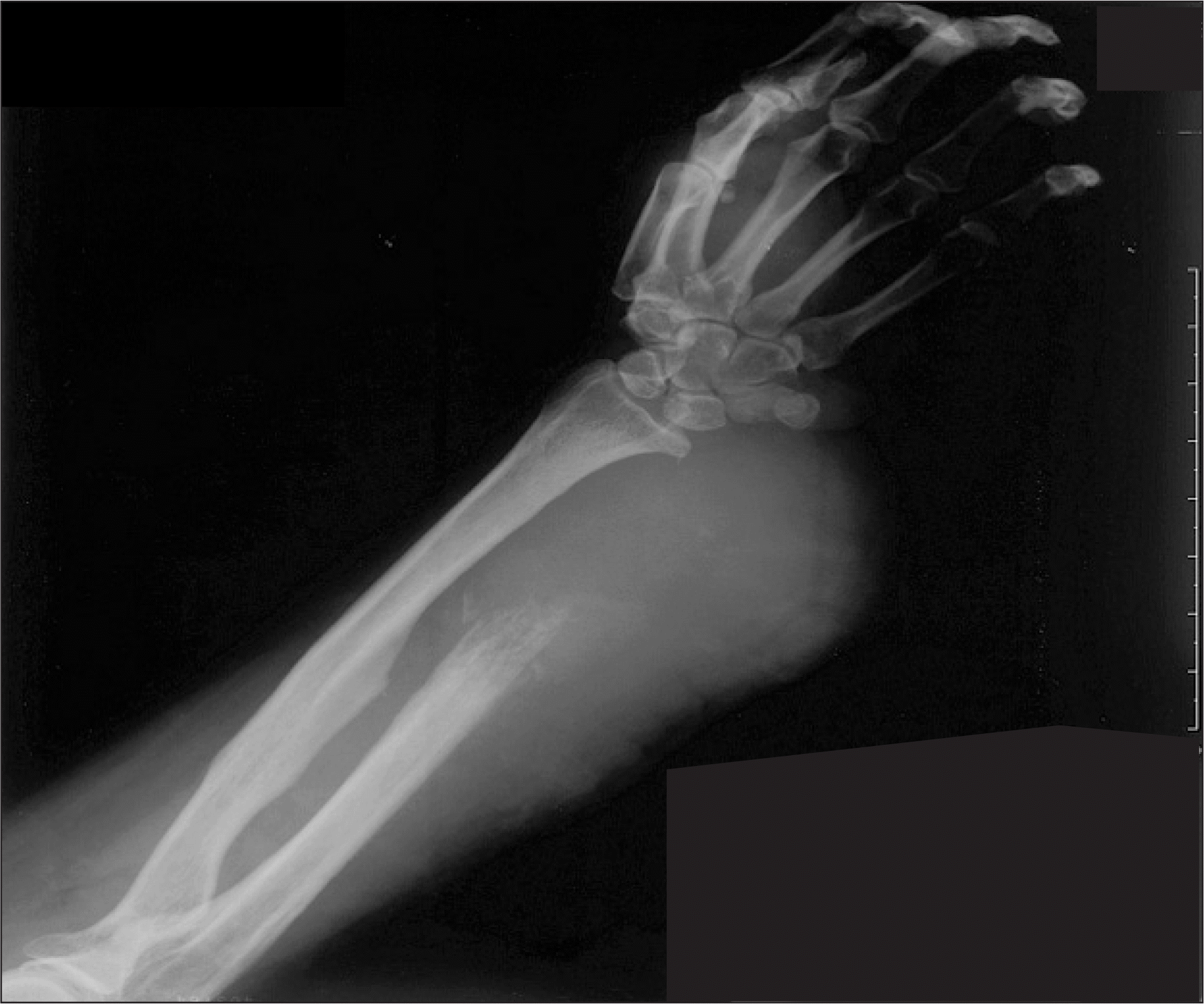
Table 1.
Summary of Patients
Table 2.
Clinical Outcomes
Table 3.
Comparison of Standard Treatment Group with Non-standard Treatment Group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download