Abstract
Advances in basic life science during the past decade have exponentially increased our understanding of the molecular bases of lung cancers and this improved insight has had great effects on diagnosis, prognosis, classification, and treatment of cancer. Similar to colorectal carcinoma, stepwise development of lung cancer has been proposed. For squamous neoplasm, dysplasia is considered to progress to invasive cancer through carcinoma in-situ in association with accumulations of genetic and epigenetic alterations. In lung adenocarcinoma, a preinvasive lesion, termed atypical adenomatous hyperplasia, progresses to an adenocarcinoma in situ, namely a bronchioloalveolar carcinoma, followed by an invasive adenocarcinoma. Currently, molecular alterations, which are involved in each transition, have been also revealed. In this review, I would like to discuss correlation of morphological features with the progression of lung adenocarcinoma with special reference to the most advanced component within the tumor.
References
1. Travis WD. Pathology and genetics of tumours of the lung, pleura, thymus, and heart. 15th ed.Lyon: IARC Press;2004.
2. Zaino RJ, Kurman RJ, Diana KL, et al. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer. 1995; 75:81–86.

3. Le Doussal V, Tubiana-Hulin M, Friedman S, et al. Prognostic value of histologic grade nuclear components of Scarff-Bloom-Richardson (SBR). An improved score modification based on a multivariate analysis of 1262 invasive ductal breast carcinomas. Cancer. 1989; 64:1914–1921.

4. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with longterm follow-up. Histopathology. 1991; 19:403–410.

6. Abbott JJ, Erickson-Johnson M, Wang X, et al. Gains of COL1A1-PDGFB genomic copies occur in fibrosarcomatous transformation of dermatofibrosarcoma protuberans. Mod Pathol. 2006; 19:1512–1518.

7. Yatabe Y, Takahashi T, Mitsudomi T. Epidermal growth factor receptor gene amplification is acquired in association with tumor progression of EGFR-mutated lung cancer. Cancer Res. 2008; 68:2106–2111.

8. Sholl LM, Yeap BY, Iafrate AJ, et al. Lung adenocarcinoma with EGFR amplification has distinct clinicopathologic and molecular features in never-smokers. Cancer Res. 2009; 69:8341–8438.

Figures
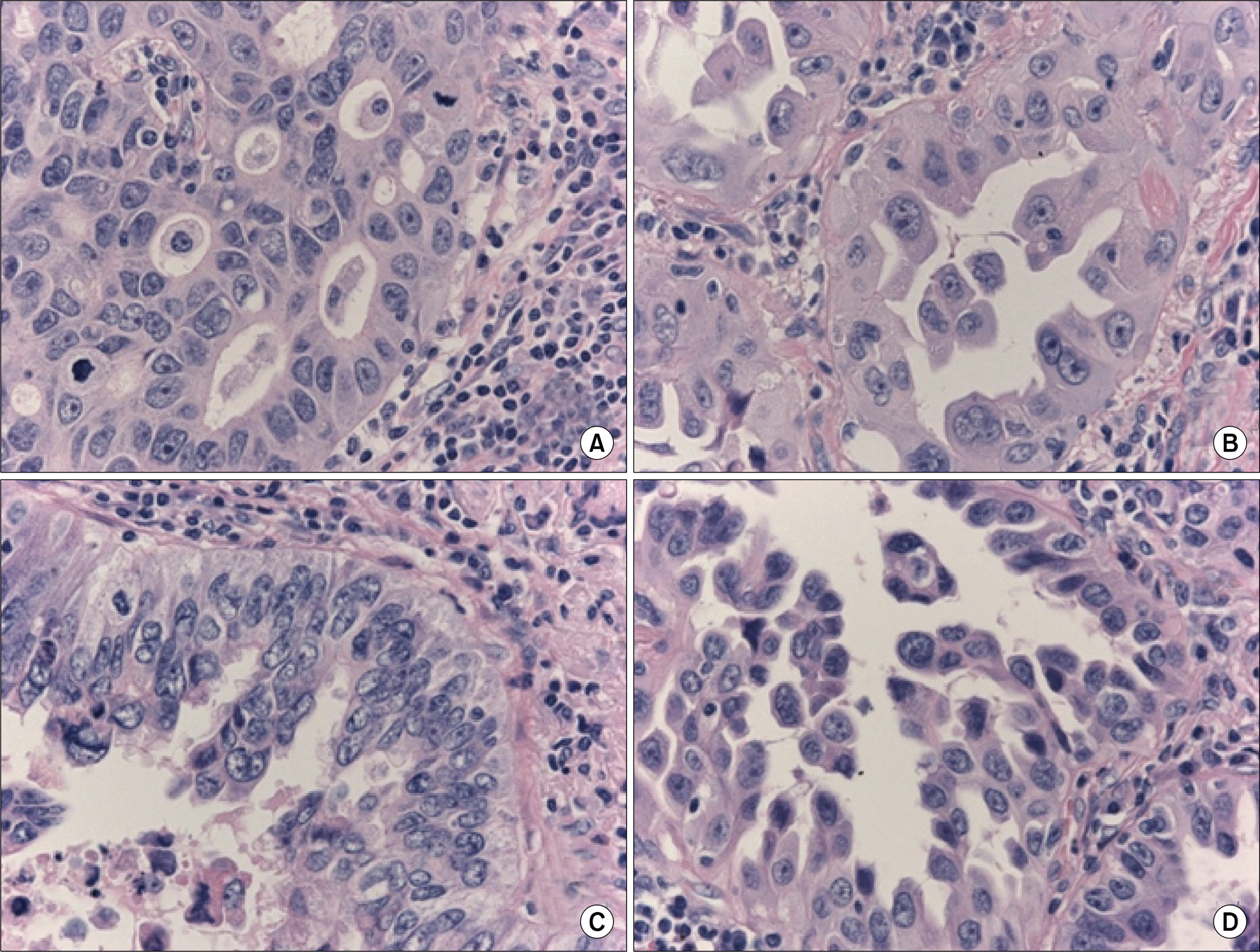
Fig. 1.
Four tissue samples from a single tumor. Morphologically, the four components were different with respect to the growth pattern and cellular atypia. All of the four components (A to D) harbored an epidermal growth factor receptor (EGFR) mutation, but EGFR amplification was detected only in components (B) and (D).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download