Abstract
Purpose
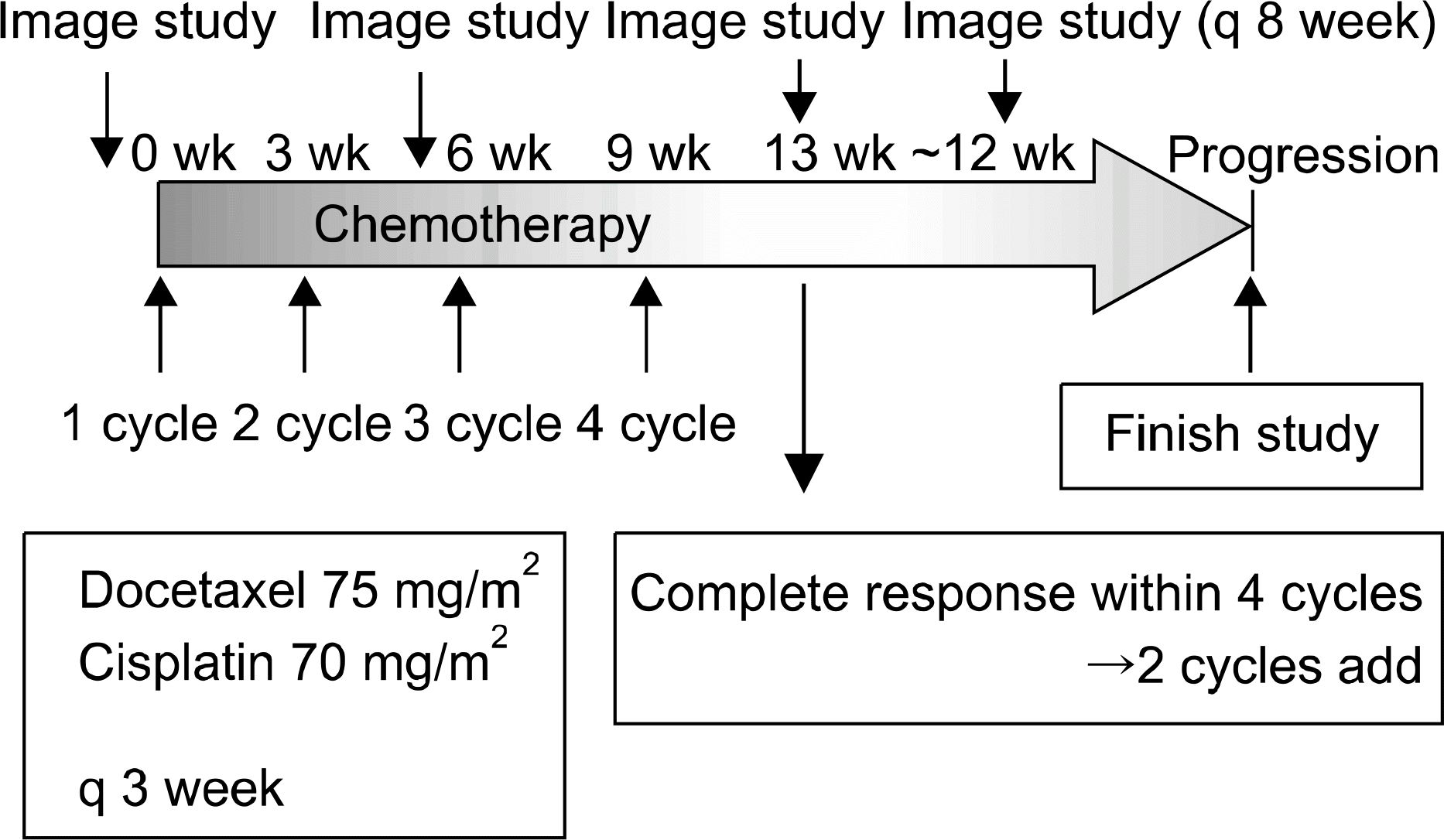
We performed a pilot study on defining duration of docetaxel and cisplatin combination chemotherapy in patients with advanced non-small cell lung cancer to evaluate its efficacy. Patients and Methods: Sixteen chemonaive patients with biopsy proven, unresectable (stage IIIB or IV) non-small cell lung cancer (NSCLC) were enrolled between January 2003 and December 2004. Treatment consisted of docetaxel (75 mg/m2/day) and cisplatin (70 mg/m2/day) every 3 week up to 4 cycles. The outcome was compared with that of a historical control group of 42 patients treated from January 1998 until December 2001, who were treated with mitomycin-C, vinorelbine, cisplatin for unresectable stage IIIB or IV NSCLC.
Results
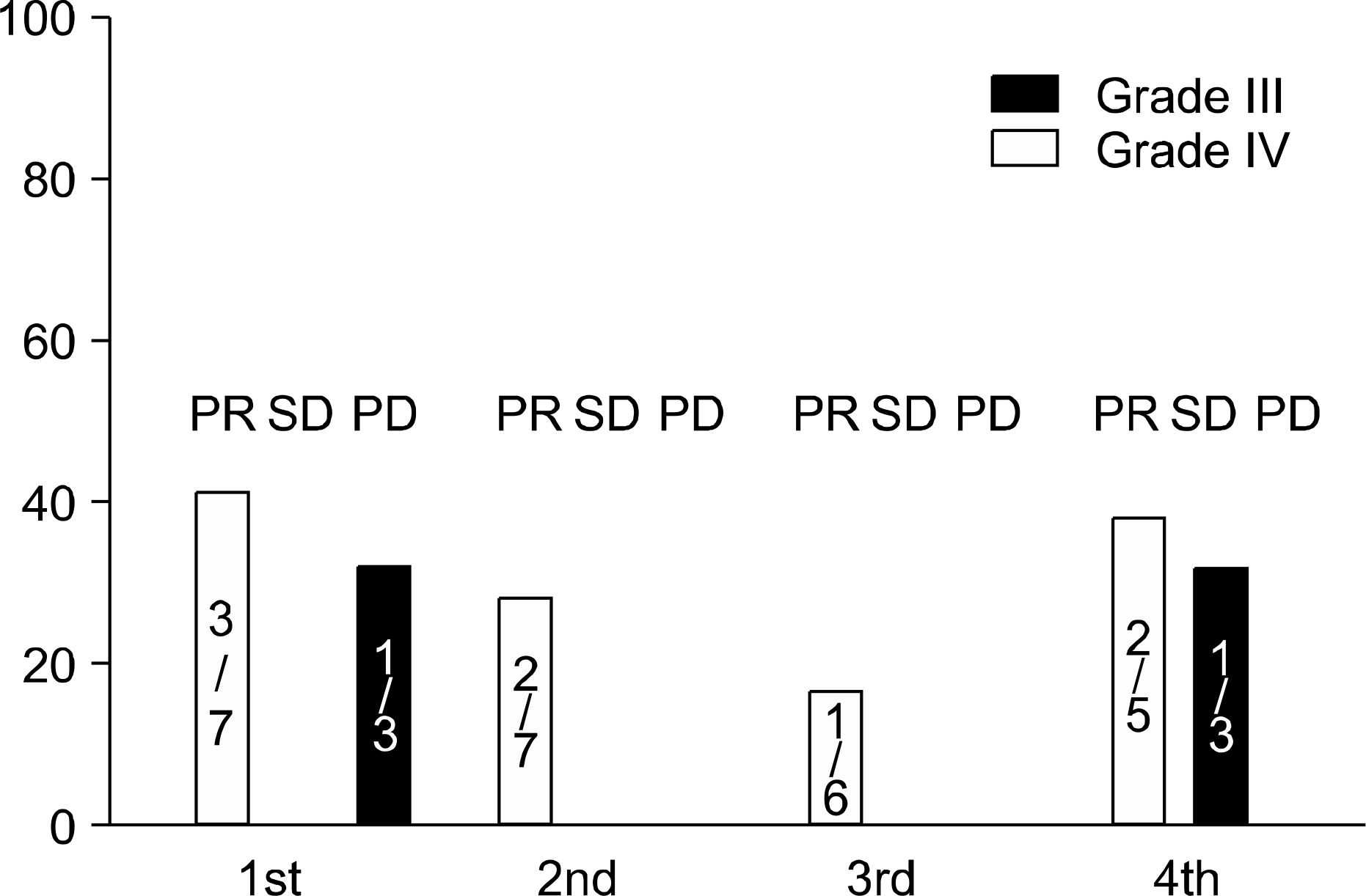
Median age was 68 (age range 43∼72). Among 16 patients, 5 patients were stage IIIB and 11 were stage IV. Fourteen patients had performance status of 0–1 and, 2 had per-formace status 2 respectively. Two patients were lost due to refusal of receiving chemotherapy. By intention-to-treat-analysis, overall response rate was 44% (C.I.: 0.19∼0.68). No complete response was noted. Median time to progression (TTP) was 144 days. Overall survival (OS) was 285 days. There is no difference in TTP & OS between docetaxel, cispatin (DP) group and mitomycin, vinorelbine, cisplatin (MVP) group statistically. Neutropenia was the most common grade III or IV toxicity; (two patients had Grade III toxicity and five Grade IV toxicity). Five patients developed febrile neutropenia, and three of neutropenia patients died due to pneumonia or septic shock. The most common non-hematologic toxicity was infection. Two of 3 patients with infection required admission.
References
1. Greene FL, Balch CM, Page DL, Haller DG, Fleming ID, Morrow M, Fritz AG. Lung. AJCC cancer staging manual: 6th edition. 2002. 167.
2. Stewart SL, King JB, Thompson TD, Friedman C, Wingo PA. Cancer mortality surveillance–United States, 1990–2000. MMWR. Surveillance summaries: Morbidity and mortality weekly report. Surveillance summaries/CDC. 53(3):). 2004; 1–108.
3. Ministry of Health and Welfare. 2002 Annual Report of the Korea Central Cancer Registry. 2003. 13.
4. 통계청. 2005 고령자 통계. 2005; 33.
5. Schrump DS, Altorki NK, Henshke CL, Carter D, Turrisi AT, Gutierrez ME. Non small cell lung cancer. DeVita VT, Hellman S, Rosenberg SA, editors. Principles and Practice of Oncology. 7th ed.Philadelphia: Lippincott-Raven;2005. p. 753–810.
6. Alberti W, Anderson G, Bartolucci A, et al. Chemotherapy in nonsmall cell lung cancer: a metaanalysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995; 311:899–909.

7. Cullen MH, Billingham LJ, Woodroffe CM, et al. Mitomycin, ifosfamide, and cisplatin in unresectable nonsmall-cell lung cancer: effects on survival and quality of life. J Clin Oncol. 1999; 17:3188–3194.

8. Thongprasert S, Sanguanmitra P, Juthapan W, Clinch J. Relationship between quality of life and clinical outcomes in advanced nonsmall cell lung cancer: Best supportive care (BSC) versus BSC plus chemotherapy. Lung Cancer. 1999; 24:17–24.

9. Helsing M, Bergman B, Thaning L, Hero U. Quality of life and survival in patients with advanced nonsmall cell lung cancer receiving supportive care plus chemotherapy with carboplatin and etoposide or supportive care only. A multicentre randomised phase III trial. Eur J Cancer. 1998; 34:1036–1044.

10. Roszkowski K, Pluzanska A, Krzakowski M, et al. A multicenter, randomized, phase III study of docetaxel plus best supportive care versus best supportive care in chemotherapynaive patients with metastatic or non-resectable localized nonsmall cell lung cancer (NSCLC). Lung Cancer. 2000; 27:145–157.

11. Gridelli C. The ELVIS trial: a phase III study of single-agent vinorelbine as first-line treatment in elderly patients with advanced nonsmall cell lung cancer. Oncologist. 2001; 6:4–7.

12. Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable nonsmall-cell lung cancer guideline: update 2003. J Clin Oncol. 2004; 22:330–353.

13. Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced nonsmall-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003; 21:3016–3024.

14. Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol. 1992; 10:282–91.

15. Rapp E, Pater JL, Willan A, et al. Chemotherapy can prolong survival in patients with advanced nonsmall-cell lung cancer-Report of a Canadian multicenter randomized trial. J Clin Oncol. 1988; 6:633–641.

16. Cartei G, Cartei F, Cantone A, et al. Cisplatin-cyclophos-phamide-mitomycin combination chemotherapy with supportive care versus supportive care alone for treatment of metastatic nonsmall-cell lung cancer. J Natl Cancer Inst. 1993; 85:794–800.

17. Smith IE, O'Brien MER, Talbot DC, et al. Duration of chemotherapy in advanced nonsmall-cell lung cancer: a randomized trial of three versus six courses of mitomycin, vinblastine, and cisplatin. J Clin Oncol. 2001; 19:1336–1343.

18. Socinski MA, Schell MJ, Peterman A, et al. .,. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV nonsmall-cell lung cancer. J Clin Oncol. 2002; 20:1335–1343.

19. Coates A, Gebski V, Bishop JF, et al. Improving the quality of life during chemotherapy for advanced breast cancer. A comparison of intermittent and continuous treatment strategies. N Engl J Med. 1987; 317:1490–1495.
20. Hickish TF, Smith IE, Mlddleton G, Nicolson M. Patient preference for extended palliative chemotherapy for nonsmall cell lung cancer. Lancet. 1995; 345:857–858.

21. Larsen H, Sorensen JB, Nielsen AL, Dombernowsky P, Hansen HH. Evaluation of the optimal duration of chemotherapy in phase II trials for inoperable nonsmall-cell lung cancer (NSCLC). Pneumologie. 1995; 6:993–7.

23. Rinaldi M, Cauchi C, Gridelli C. First line chemotherapy in advanced or metastatic NSCLC. Ann Oncol. 2006; 17(suppl 5):64–67.

24. Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with nonsmall-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000; 18:2095–103.

25. Engels FK. and Verweij J. Docetaxel administration schedule: from fever to tears? A review of randomised studies. Eur J Cancer. 2005; 41:1117–26.
Fig. 2.
The median remision duration of DP gruop is 153 days. That of MVP group is 216 days. But there is not statistically significance (p value=0.327). DP: docetaxel and cisplatin, MVP: mitomycin-C and vinorelbine, cisplatin.
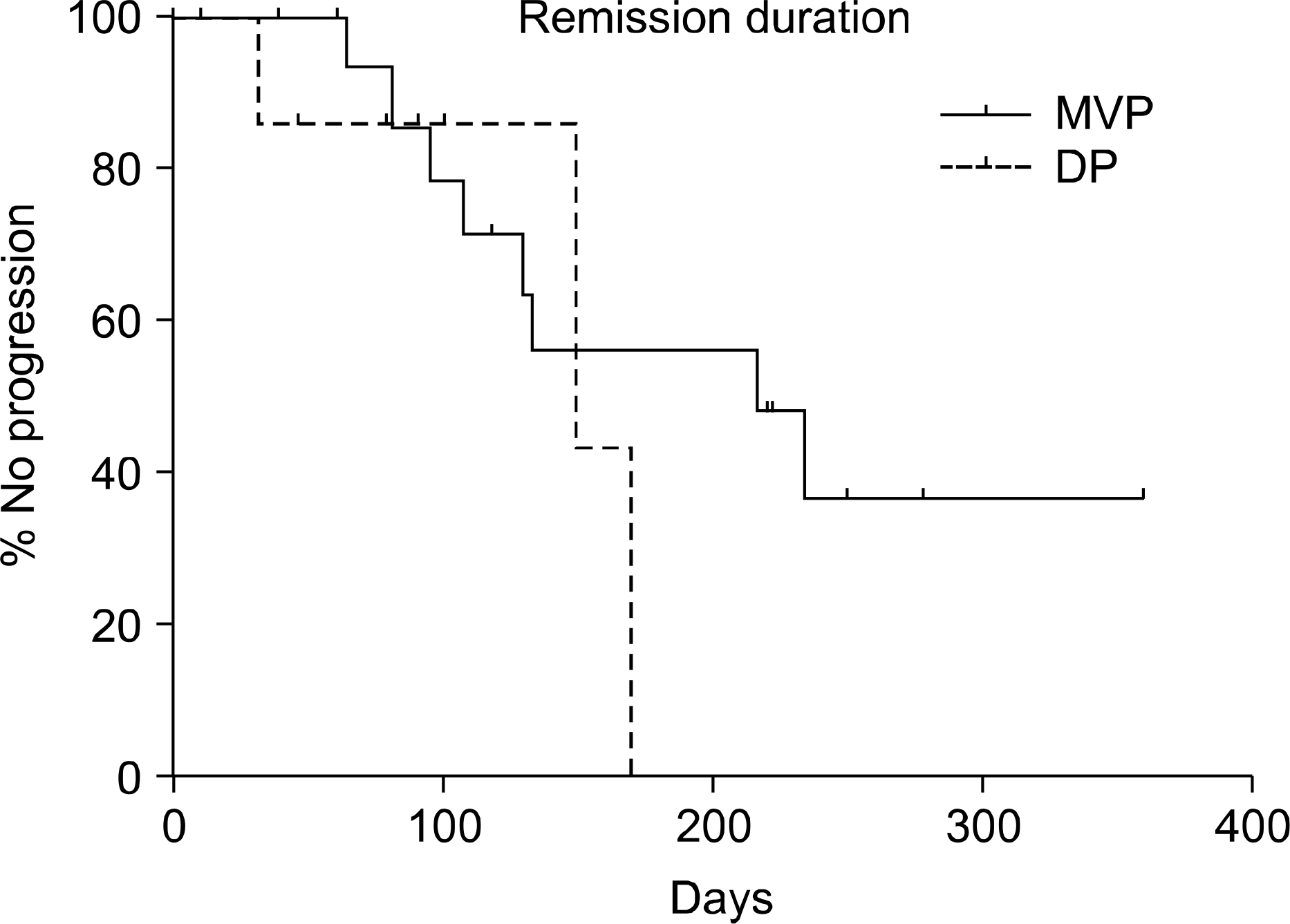
Fig. 3.
The median time to progression (TTP) of DP gruop is 144 days. That of MVP group is 211 days. But there is not statistically significance (p value=0.49). DP: docetaxel and cisplatin, MVP: mitomycin-C and vinorelbine, cisplatin.
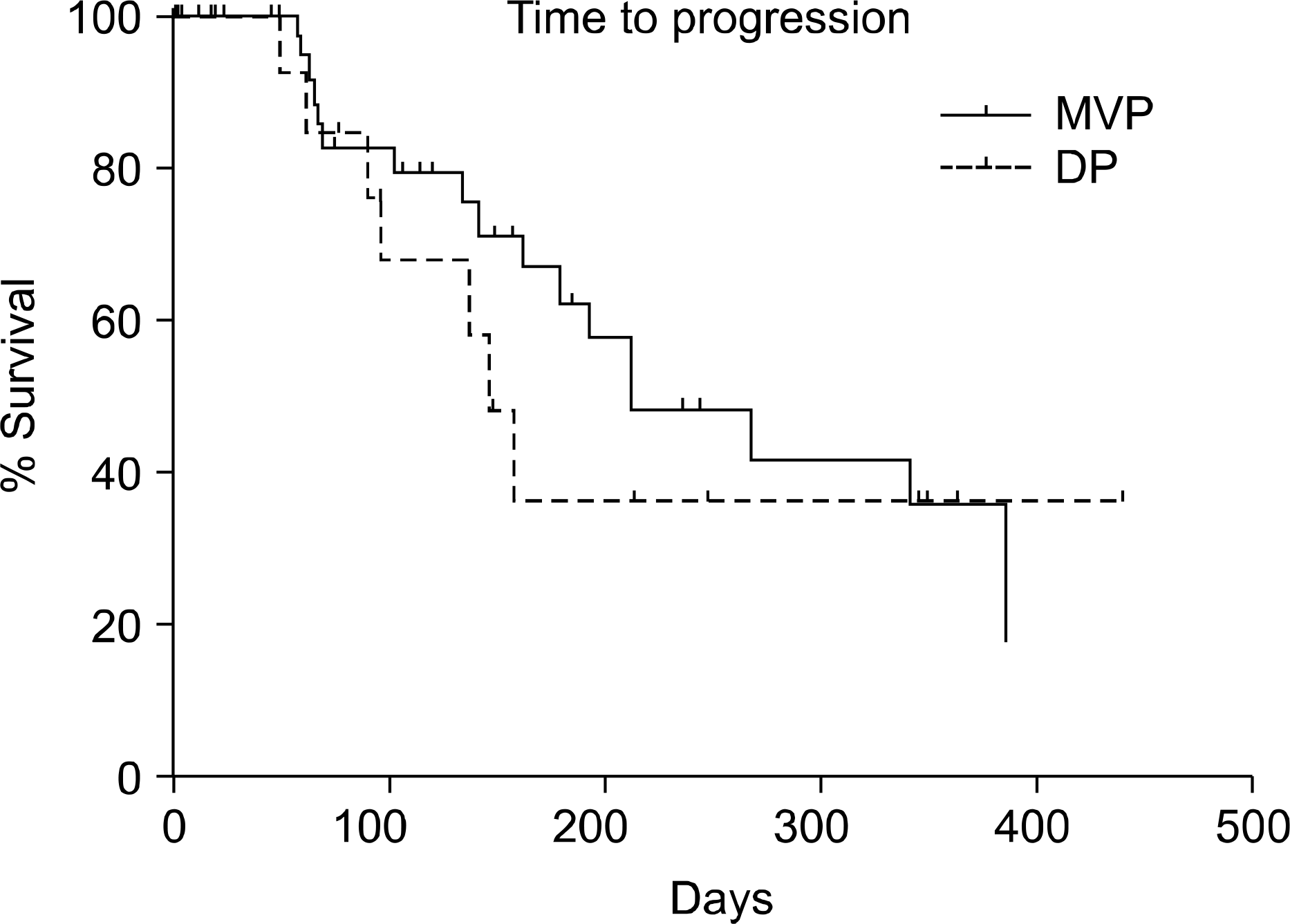
Fig. 4.
The median overall survival (OS) of DP gruop is 285 days. That of MVP group is 410 days. But there is not statistically significance (p value=0.16). DP: docetaxel and cisplatin, MVP: mitomycin-C and vinorelbine, cisplatin.
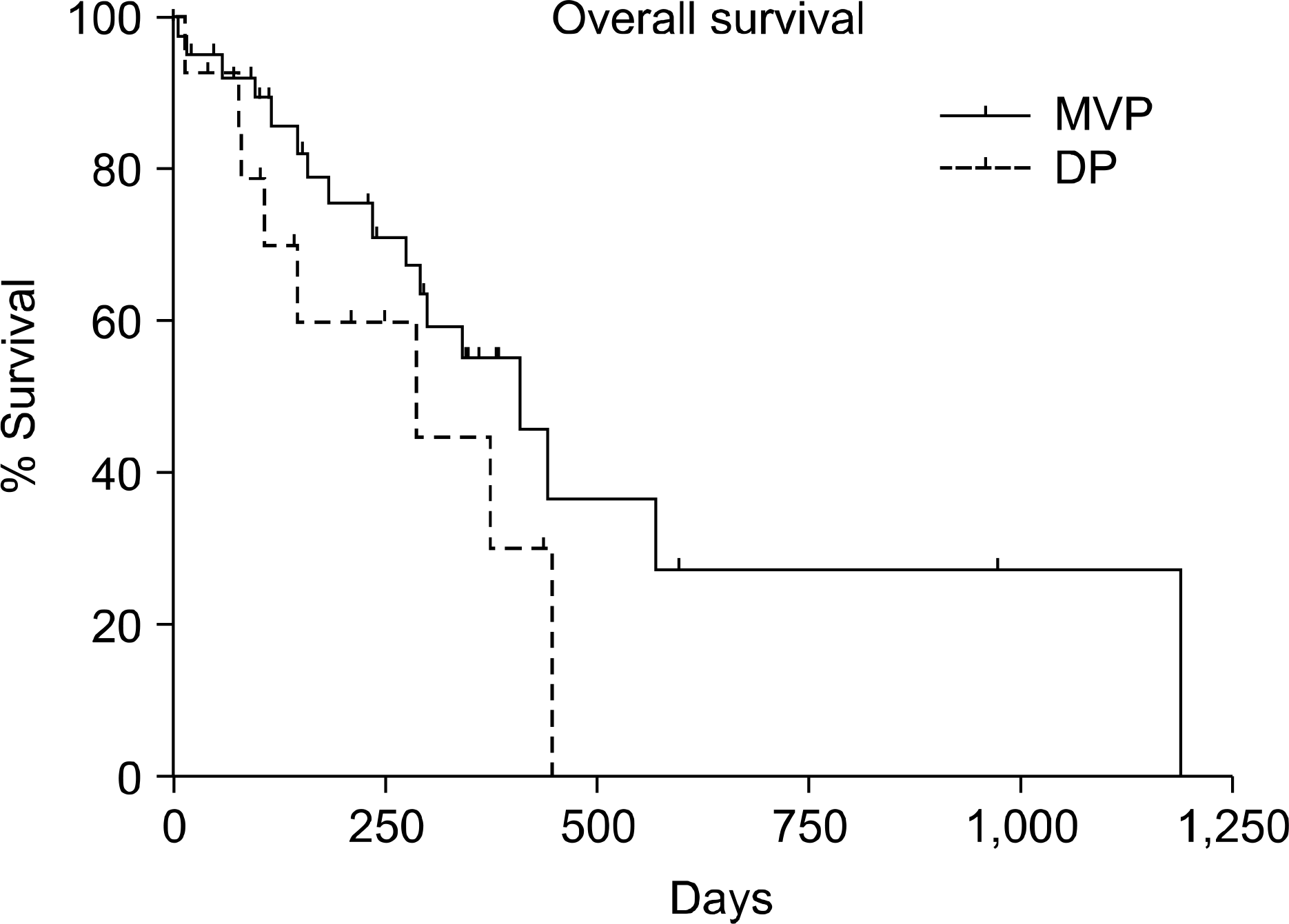
Table 1.
Baseline Characteristics of the Patients (n=16)
Table 2.
Tumor Response to 4 Cycles of Docetaxel and Cisplatin Chemotherapy (n=16)
| Response | No. of patients (%) |
|---|---|
| Complete response | 0 (0%) |
| Partial response | 7 (44%) |
| Stable disease | 5 (31%) |
| Progressive disease | 2 (13%) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download