Abstract
Purpose:
We evaluated the optimal combination of thoracic radiotherapy with chemotherapy in patients with limited-stage small cell lung cancer (L-SCLC).
Materials and Methods:
We retrospectively analyzed the data of 95 patients with L-SCLC who completed the planned thoracic radiotherapy combined with chemotherapy between January 1998 and March 2004, Thoracic radiotherapy was done with conventional fractionation to the median dose of 60Gy. Radiotherapy was combined with chemotherapy conc䴸rrently (n=67), alternating (n二 19)ôor sequentially (n=9). Chemotherapy consisted of EP or EC (etoposide 100 mg/m2, cisplatin 60 mg/m2 or carboplatin 5~6xAUC), The median cycle of chemotherapy was 6 with the range of 2 to 8.
Results:
The median survival of all 95 patients was 20 months and 2-, 3-, and 5-year overall survival rate was 39%, 26%, and 19%, respectively. Radiation dose above 55 Gy did not show better survival result than dose below 55 Gy (p=0.59). The median survival and 5-year survival rate of 67 patients with concurrent chemoradiation was 23 months and 24% while those of 28 patients with alternating or sequential chemoradiation was 16 months and 8%, respectively (p=0.007). Concurrent thoracic radiotherapy combined after 2 cycles of chemotherapy showed the best survival res䴸Its among the combination methods (p=0.29). The survival was improved in patients with chemotherapy more than 5 cycles comparing to patients with less than 5 (p=0.03). Patients with PCI showed the median survival of 43 months and 5-year survival rate of 35% vs. 18 months and 16% in patients without PCI, respectively (ρ=0Ό2). In multivariate analysis, the concurrent chemoradiation was the only significant prognostic factor affecting to the survival.
Conclusion:
Concurrent chemoradiation after 2 cycles of chemotherapy showed the best survival results in our study group. F䴸II dose of chemotherapy to 6 cycles needed to be proceeded in tolerable patients. PCI can be recommended to the patients with complete response after chemoradiation. (J Lung Cancer 2006;5{1):17-22)
REFERENCES
1.Perry MC., Hendon JE., Eaton WL, et al. Thoracic radiation therapy added to chemotherapy for small-cell lung cancer: an update of Cancer and Leukemia Group B Study Group 8083. J Clin Oncol. 1998. 16:2466–2467.
2.Pignon JP., Arriagada R., Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer, N Engl J Med. 1992. 327:1618–1624.
3.Warde P: Payne D. Dose thoracic irradiation improve survival and local control in limited-stage small-cell lung carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992. 10:890–895.
4.Parvesh K. The role of thoracic radiotherapy in the management of limited-stage small cell lung cancer: past, present, and future. Chest. 1997. 112:259S–265S.
5.Turrisi AT., Kim K., Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated with concurrently with cisplatin and etoposide. N Engl J Med. 1999. 340:265–27. L.
6.Carney DN., Mitchell JB: Kinsella TJ. In vitro radiation and chemotherapy sensitivity of established cell lines of human small cell lung cancer and its large cell morphologic variants. Cancer Res. 1983. 43:2806–2811.
7.Choi NC., Herdon J., Rosenmann J, et al. Long-term survival data from CALGB 8837: radiation dose escalation and concurrent chemotherapy (CT) in limited stage small cell lung cancer (LD-SCLC). Possible radiation dose-survival relationship (Abstract). Proc ASCO. 2002. 21:298a.
8.Roof KS., Fidas P., Lynch TJ., Ancukiewicz M: Choi NC. Radiation dose escalation in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003. 57:701–708.

9.Miller KL., Marks LB., Sibley GS, et al. Routine use of aj> proximately 60 Gy once-daily thoracic irradiation for patients with limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2003. 56:355–359.
10.Bogart JA., Herdon JE: Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of cancer and leukemia group B study 39808. Int J Radiat Oncol Biol Phys. 2004. 59:460–468.

11.Murray N., Coy P., Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. J Clin Oncol. 1993. 11:336–344.
12.Liengswangwong V: Bonner JA., Shaw EG, et al. Limited-stage small-cell cancer: patterns of intrathoracic recurrence and the implications for thoracic radiotherapy. J Clin Oncol. 1994. 12:496–502.
13.Schild SE., Bonner JA., Shanahan TG, et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiography with twice-daily radiotherapy in limited-stage small-cell lung cancer, Int J Radiat Oncol Biol Phys. 2004. 59:943–951.
14.Yeo S: Cho M., Kim S., Kim K., Kim J. Twice daily radiation therapy plus concurrent chemotherapy for limited-stage small cell lung cancer. J Korean Soc Ther Radiol Oncol. 2006. 24:96–102.
15.Blum R., MacManus MP., Rischin D., Michael M., Hicks RJ. Impact of positron emission tomography on the management of patients with small-cell lung cancer. Am J Clin Oncol. 2004. 27:164–171.

16.Auperin A., Aniagada R., Pigna JP, et al. Prophylactic cranial irriadiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999. 341:476–484.
Fig. 1.
The survival curves according to the timing of thoracic radiotherapy in patients with concurrent chemoradiation (n=67) was shown.
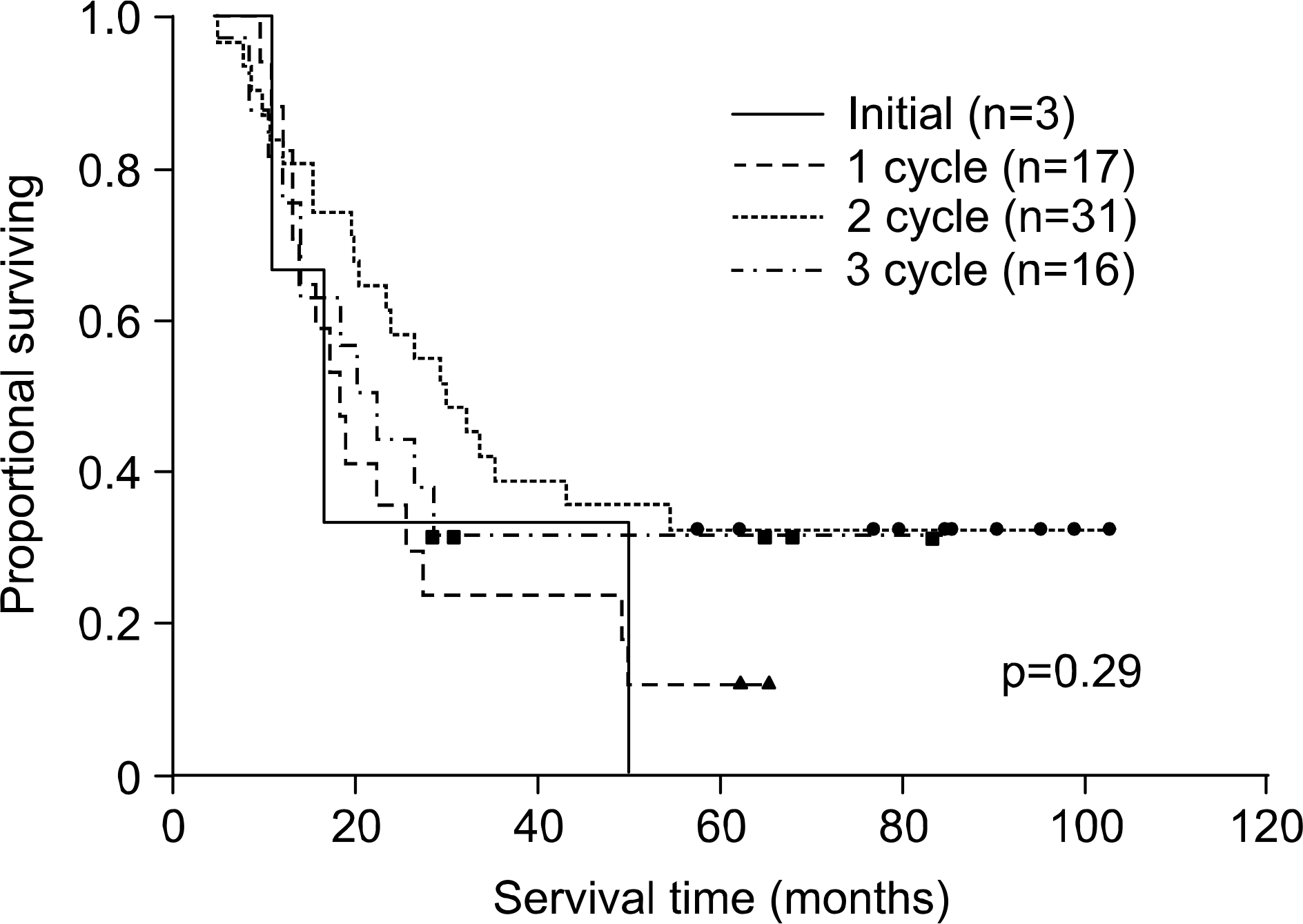
Table 1.
Patients Characteristics (n=95)
Table 2.
Univariate Analysis of Prognostic Factors on Survival
Table 3.
Multivariate Analysis on Survival
| Parameters | ρ value |
|---|---|
| Age-60 | 0.81 |
| Gender | 0.72 |
| Tx. duration-23 weeks | 0.30 |
| CCRT | 0. 01 |
| RT dose-50 Gy | 0.59 |
| PCI | 0.08 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download