Abstract
Locally advanced NSCLC is a heterogenous group of bronchogenic malignancies that are traditionally thought to be unresectable without overt distant metastasis or malignant pleural effusion. The mainstay of treatment for this class of diseases until the early 1990s was radiation alone, which resulted in a dismal outcome. The new technologies in radiation therapy (e.g. 3D-CRT) and the shift in paradigm (e.g. omission of ENI) have enabled the dose-escalation, which translated to improved outcome compared to the conventional radiotherapy using 2-D planning. The trials combining chemotherapy with radiotherapy, first sequentially, then concurrently, have changed the standard of care for patients with good functional status to concurrent chemᄋradiation. Some studies have shown survival benefits to adding consolidative systemic therapy with concurrent chemoradiation. We will outline the development of the current treatment standard of locally advanced NSCLC and present selected topics 䴸ndergoing active research to forecast the next generation of NSCLC therapy. (J Lung Cancer 2006;5(1):1-16)
REFERENCES
1.The World Health Organization (WHO). The World Health Report;. 2003.
2.Perez CA., Pajak TF., Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer. 1987. 59:1874–1881.

3.Petrovich Z., Stanley K., Cox JD., Paig C. Radiotherapy in the management of locally advanced lung cancer of all cell types: final report of randomized trial. Cancer. 1981. 48:1335–1340.

4.Roswit B., Patno ME., Rapp R, et al. The survival of patients with inoperable lung cancer: a large-scale randomized study of radiation therapy versus placebo. Radiology. 1968. 90:688–697.

5.Payne DG. Non-small-cell lung cancer: should unresectable stage III patients routinely receive high-dose radiation therapy? J Clin Oncol. 1988. 6:552–558.

6.Furuse K., Fukuoka M., Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999. 17:2692–2699.

7.Curran WJ. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemoradiation for patients with unresected stage III nsclc: RTOG 9410. Proc Am Soc Clin Oncol. 2003. 22:621.
8.Foumel P., Robinet G., Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne dOncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol. 2005. 23:5910–5917.
9.Zatloukal P., Petruzelka L., Zemanova M, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004. 46:87–98.

10.Fischer JJ., Moulder JE. The steepness of the dose-response curve in radiation therapy. Theoretical considerations and experimental results. Radiology. 1975. 117:179–184.
11.Fletcher GR Clinical dose response curves of human malignant epithelial tumours. Br J Radiol. 1973. 46:151.
12.Mantravadi RV: Gates JO., Crawford JN: Bajpai D., Trenkner JD., Jordan LN. Unresectable non-oat cell carcinoma of the lung: definitive radiation therapy. Radiology. 1989. 172:851–855.

13.Moore JV., Hendry JH: Hunter RD. Dose-incidence curves for tumour control and normal tissue injury, in relation to the response of clonogenic cells. Radiother Oncol. 1983. 1:143–157.

14.Munro TR., Gilbert CW. The relation between tumour lethal doses and the radiosensitivity of tumour cells. Br J Radiol. 1961. 34:246–251.

15.Thames HD Jr., Peters 니: Spanos W Jr., Fletcher GF. Dose response of squamous cell carcinomas of the upper respiratory and digestive tracts. Br J Cancer Suppl. 1980. 41(Suppl 4):35–38.
16.Fuks Z., Leibel SA., Kutcher GJ: Mohan R: Ling CC. Three-dimensional conformal treatment: a new frontier in radiation therapy. Important Adv Oncol. 1991. 151–172.
17.Goitein M: Abrams M. Multi-dimensional treatment planning: I. Delineation of anatomy. Int J Radiat Oncol Biol Phys. 1983. 9:777–787.
18.McShan D: Haumann D: Glicksman AS. A new interactive computerized data base and retrieval system. Comput Programs Biomed. 1979. 9:284–292.
19.Hayman JA., Martel MK: Ten Haken RK, et al. Dose escalation in non-small-cell lung cancer using three-dimensional conformal radiation therapy: update of a phase I trial. J Clin Oncol. 2001. 19:127–136.

20.Rosenzweig KE., Mychalczak B., Fuks Z, et al. Final report of the 70.2-Gy and 75.6-Gy dose levels of a phase I dose escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable non-small cell lung cancer. Cancer J. 2000. 6:82–87.
21.Mehta M., Scrimger R., Mackie R., Paliwal B: Chappell R., Fowler J. A new approach to dose escalation in non-smallcell lung cancer. Int J Radiat Oncol Biol Phys. 2001. 49:23–33.

22.Perez CA., Stanley K., Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980. 45:2744–2753.

23.Arriagada R: Le Chevalier T., Quoix E, et al. ASTRO (American Society for Therapeutic Radiology and Oncology) plenary: Effect of chemotherapy on locally advanced nonsmall cell lung carcinoma: a randomized study of 353 patients. GETCB (Groupe d'Etude et Traitement des Cancers Bronchiques), FNCLCC (Federation Nationale des Centres de Lutte contre le Cancer) and the CEBI trialists. Int J Radiat Oncol Biol Phys. 1991. 20:1183–1190.
24.Cox JD., Azamia N'Byhardt RW: Shin KH., Emami B., Pajak TF. A randomized phase I/II trial of hyperfractionated radiation therapy with total doses of 60.0 Gy to 79.2 Gy: possible survival benefit with greater than or equal to 69.6 Gy in favorable patients with Radiation Therapy Oncology Group stage III non-small-cell lung carcinoma: report of Radiation Therapy Oncology Group 83-11. J Clin Oncol. 1990. 8:1543–1555.

25.Hazuka MB., Turrisi AT 3rd., Lutz ST, et al. Results of high-dose thoracic irradiation incorporating beam's eye view display in non-small cell lung cancer; a retrospective multivariate analysis. Int J Radiat Oncol Biol Phys. 1993. 27:273–284.

26.Sibley GS., Mundt AJ., Shapiro C, et al. The treatment of stage III nonsmall cell lung cancer using high dose conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1995. 33:1001–1007.

27.Armstrong JG., Zelefsky MJ., Leibel SA, et al. Strategy for dose escalation using 3-dimensional conformal radiation therapy for lung cancer. Ann Oncol. 1995. 6:693–697.

28.Robertson JM., Ten Haken RK., Hazuka MB, et al. Dose escalation for non-small cell lung cancer using conformal radiation therapy. Int J Radiat Oncol Biol Phys. 1997. 37:1079–1085.

29.Rosenman JG., Halle JS., Socinski MA, et al. High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys. 2002. 54:348–356.

30.Socinski MA: Morris DE., Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol. 2004. 22:4341–4350.
31.Bradley J: Graham MV., Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005. 61:318–328.
32.Curran WJ Jr., Moldoisky PJ., Solin LJ. Analysis of the influence of elective nodal irradiation on postirradiation pulmonary function. Cancer. 1990. 65:2488–2493.

33.Rosenzweig KE., Sim SE., Mychalczak B., Braban LE., Schindelheim R., Leibel SA. Elective nodal irradiation in the treatment of non-small-cell lung cancer with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2001. 50:681–685.

34.Yorke ED., Wang L., Rosenzweig KE., Mah D., Paoli JB., Chui CS. Evaluation of deep inspiration breath-hold lung treatment plans with Monte Carlo dose calculation. Int J Radiat Oncol Biol Phys. 2002. 53:1058–1070. ,.

35.Lax I: Blomgren H., Naslund I: Svanstrom R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994. 33:677–683.
36.Wulf J., Hadinger U: Oppitz U., Olshausen B: Flentje M. Stereotactic radiotherapy of extracranial targets: CT-simulation and accuracy of treatment in the stereotactic body frame. Radiother Oncol. 2000. 57:225–236.

37.Negoro Y., Nagata Y., Aoki T, et al. The effectiveness of an immobilization device in conformal radiotherapy for lung tumor: reduction of respiratory tumor movement and evaluation of the daily setup accuracy. Int J Radiat Oncol Biol Phys. 2001. 50:889–898.

38.Lohr F., Debus J., Frank C, et al. Noninvasive patient fixation for extracranial stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1999. 45:521–527.

39.Herfarth KK., Debus J., Lohr F, et al. Extracranial stereotactic radiation therapy: set-up accuracy of patients treated for liver metastases. Int J Radiat Oncol Biol Phys. 2000. 46:329–335.

40.Keall PJ., Kini VR., Vedam SS., Mohan R. Potential radiotherapy improvements with respiratory gating. Australas Phys Eng Sci Med. 2002. 25:1–6.

41.Whyte RI., Crownover R., Murphy MJ, et al. Stereotactic radiosurgery for lung tumors: preliminary report of a phase I trial. Ann Thorac Surg. 2003. 75:1097–1101.

42.Saunders M: Dische S., Barrett A: Harvey A., Gibson D., Parmar M. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet. 1997. 350:161–165.
43.Belani CP., Wang W., Johnson DH, et al. Phase III study of the Eastern Cooperative Oncology Group (ECOG 2597): induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non-small-cell lung cancer. J Clin Oncol. 2005. 23:3760–3767.

44.Sause W., Kolesar P., Taylor SI, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest. 2000. 117:358–364.
45.Curran WJ Jr. Phase III Comparison of Sequential vs. Concurrent Chemo-Radiation For Patients With Unresected Stage Hi Non-Small Cell Lung Cancer (NSCLC): Report of Radiation Therapy Oncology Group (RTOG) 9410. Proc Am Soc Clin Oncol. 2000.
46.Dillman RO., Seagren SL., Proper! KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990. 323:940–945.

47.Dillman RO., Herndon J., Seagren SL., Eaton WL Jr.., Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996. 88:1210–1215.
48.Le Chevalier T., Arriagada R., Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst. 1991. 83:417–423.

49.Le Chevalier T., Arriagada R., Tarayre M, et al. Significant effect of adjuvant chemotherapy of survival in locally advanced non-small-cell lung carcinoma. J Natl Cancer Inst. 1992. 84:58–59.
50.Steel GG., Peckham MJ. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int J Radiat Oncol Biol Phys. 1979. 5:85–91.
52.Herscher LL: Cook JA: Pacelli R., Pass HI., Russo A., Mitchell JB. Principles of chemoradiation: theoretical and practical considerations. Oncology (Williston Park). 1999. 13((10 Suppl 5):):11–22.
53.Sause WT., Scott C., Taylor S, et al. Phase II trial of combination chemotherapy and irradiation in non-small-cell lung cancer, Radiation Therapy Oncology Group 88-04. Am J Clin Oncol. 1992. 15:163–167.

54.Albain KS., Crowley JJ., Turrisi AT 3rd, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage II1B non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002. 20:3454–3460.
55.Gandara DR., Chansky K: Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol. 2003. 21:2004–2010.

56.Huber ., Schmidt ., Flentje , et al. Induction chemotherapy and following simultaneous radio/chemotherapy versus induction chemotherapy and radiotherapy alone in inoperable NSCLC (Stage ΙΙΙΛ/Π1Β) Proc Am Soc Clin Oncol. 2003. 22:622.
57.Vokes ., Herndon ., Kelley ., Watson . Cicchetti: Green. Induction chemotherapy followed by concomitant chemoradiotherapy versus chemoradiotherapy alone for regionally advanced unresectable non-small cell lung cancer (NSCLC): initial analysis of a randomized phase III trial. J Clin Oncol. 2004. 22(14S):
58.Belani CP., Choy H., Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005. 23:5883–5891.

59.Bonomi P., Kim K., Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000. 18:623–631.

60.Schiller JH., Harrington D., Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002. 346:92–98.

61.Vokes EE., Herndon JE 2nd., Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol. 2002. 20:4191–4198.

62.Scagliotd GV., De Marinis F., Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002. 20:4285–4291.
63.Rose!) R., Gatzemeier U., Betticher DC, et al. Phase III randomised trial comparing paditaxel/carboplatin with paclitaxel/cisplatin in patients with advanced non-small-cell lung cancer: a cooperative multinational trial. Arm Oncol. 2002. 13:1539–1549.
64.Kelly K., Crowley J., Bunn PA Jr, et al. Randomized phase 10 trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced nonsmall-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001. 19:3210–3218. ,.
65.Fossella F: Pereira JR., von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003. 21:3016–3024.
66.Georgoulias V: Papadakis E: Alexopoulos A, et al. Platinum-based and non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a randomised multicentre trial. Lancet. 2001. 357:1478–1484.
67.Gridelli C., Gallo C., Shepherd FA, et al. Gemcitabine plus vinorelbine compared with cisplatin plus vinorelbine or cisplatin plus gemcitabine for advanced non-small-cell lung cancer: a phase 111 trial of the Italian GEMVIN Investigators and the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003. 21:3025–3034.
68.Brizel DM., Wasserman TH., Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000. 18:3339–3345.

69.Antonadou D., Coliarakis N., Synodinou M, et al. Randomized phase III trial of radiation treatment +/– amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol Biol Phys. 2001. 51:915–922.

70.Komaki R., Lee JS., Milas L, et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small-cell lung cancer: report of a randomized comparative trial. Int J Radiat Oncol Biol Phys. 2004. 58:1369–1377.

71.Leong SS: Tan EH., Fong KW, et al. Randomized doubleblind trial of combined modality treatment with or without amifostine in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2003. 21:1767–1774.
72.Movsas B: Scott C., Langer C, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98-01. J Clin Oncol. 2005. 23:2145–2154.
73.Timmerman R., Papiez L., McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003. 124:1946–1955.
74.McGarry RC: Papiez L: Williams M: Whitford T., Timmerman RD. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys. 2005. 63:1010–1015.
75.Onishi H., Araki T., Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage 1 nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004. 101:1623–1631.
76.Onishi H., Kuriyama K: Komiyama T, et al. Clinical outcomes of stereotactic radiotherapy for stage I non-small cell lung cancer using a novel irradiation technique: patient self-controlled breath-hold and beam switching using a combination of linear accelerator and CT scanner. Lung Cancer. 2004. 45:45–55.

77.Nagata Y., Negoro Y., Aoki T, et al. Clinical outcomes of 3D conformal hypofractionated single high-dose radiotherapy for one or two lung tumors using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2002. 52:1041–1046.

78.Nagata Y., Takayama K., Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005. 63:1427–1431.

79.Mendelsohn J., Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003. 21:2787–2799.

80.Ozanne B., Richards CS., Hendler F: Bums D: Gusterson B. Over-expression of the EGF receptor is a hallmark of squamous cell carcinomas. J Pathol. 1986. 149:9–14.

81.Fukuoka M., Yano S., Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial). J Clin Oncol. 2003. 21:2237–2246.
82.Kris MG., Natale RB., Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. Jama. 2003. 290:2149–2158.
83.Giaccone G., Herbst RS., Manegold C., el al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial-INTACT 1. J Clin Oncol. 2004. 22:777–784.
84.Herbst RS., Giaccone G., Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced nonsmall-cell lung cancer: a phase III trial-INTACT 2. J Clin Oncol. 2004. 22:785–794.
85.Bonner JA: Giralt J: Harari PM, et al. Cetuximab prolongs survival in patients with locoregionally advanced squamous cell carcinoma of head and neck: A phase III study of high dose radiation therapy with or without cetuximab. Proc Am Soc Clin Oncol. 2004. 22(14S):): (abst 5507).
86.Patz EF Jr., Lowe VJ: Hoffman JM, et al. Focal pulmonary abnormalities: evaluation with F-18 fluorodeoxyglucose PET scanning. Radiology. 1993. 188:487–490.

87.Lowe VJ: Fletcher JW., Gobar L, et al. Prospective investigation of positron emission tomography in lung nodules. J Clin Oncol. 1998. 16:1075–1084.
88.Gould MK., Maclean CC., Kuschner WG., Rydzak CE., Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. Jama. 2001. 285:914–924.
89.Gambhir SS: Czemin J: Schwimmer J: Silverman DH., Coleman RE., Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001. 42((Suppl 5):):1S–93S.
90.Pieterman RM., van Putten JW., Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000. 343:254–261.

91.Saunders CA., Dussek JE: O'Doherty MJ., Maisey MN. Evaluation of fluorine-18-fluorodeoxyglucose whole body positron emission tomography imaging in the staging of lung cancer. Ann Thorac Surg. 1999. 67:790–797.

92.Bueno R., Richards WG., Swanson SJ, et al. Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg. 2000. 70:1826–1831.

93.Voltolini L., Luzzi L., Ghiribelli C., Paladini P., Di Bisceglie M: Gotti G. Results of induction chemotherapy followed by surgical resection in patients with stage IIIA (N2) non-small cell lung cancer: the importance of the nodal down-staging after chemotherapy. Eur J Cardiothorac Surg. 2001. 20:1106–1112.

94.Nestle U., Walter K., Schmidt S, et al. 18F-deoxyglucose positron emission tomography (FDG-PET) for the planning of radiotherapy in lung cancer: high impact in patients with atelectasis. Int J Radiat Oncol Biol Phys. 1999. 44:593–597.

95.Munley MT., Marks LB., Scarfone C, et al. Multimodality nuclear medicine imaging in three-dimensional radiation treatment planning for lung cancer: challenges and prospects. Lung Cancer. 1999. 23:105–114.

96.Vanuytsel LJ., Vansteenkiste JF., Stroobants SG, et al. The impact of (18)F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) lymph node staging on the radiation treatment volumes in patients with non-small cell lung cancer. Radiother Oncol. 2000. 55:317–324.

97.Bradley J., Thorstad WL., Mutic S, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004. 59:78–86.

98.Ichiya Y., Kuwabara Y., Sasaki M, et al. A clinical evaluation of FDG-PET to assess the response in radiation therapy for bronchogenic carcinoma. Ann Nucl Med. 1996. 10:193–200.

99.Choi NC., Fischman AJ., Niemierko A, et al. Dose-response relationship between probability of pathologic tumor control and glucose metabolic rate measured with FDG PET after preoperative chemoradiotherapy in locally advanced nonsmall-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002. 54:1024–1035.

Fig. 1.
Three-dimensional treatment plan, (A) Coronal section through isᄋcenter of a typical three-dimensional treatment plan isᄋdose distribution. (B) Axial section at 3.5 cm superior to isocenter for the same patient. The planning target volume is outlined in yellow. The patient had T3 NO norismal! cell lung cancer based on disease extension into the right mainstem bronchus. Based on these isodose distributions, the doses were estimated as follows; subcarina, 8,000 cGyæ ispsilateral inferior mediastinum. 4,000 cGy: contralateral inferior mediastinum, 1,000 cGy: superior mediastinum and supraclavicular region 0 Gy.
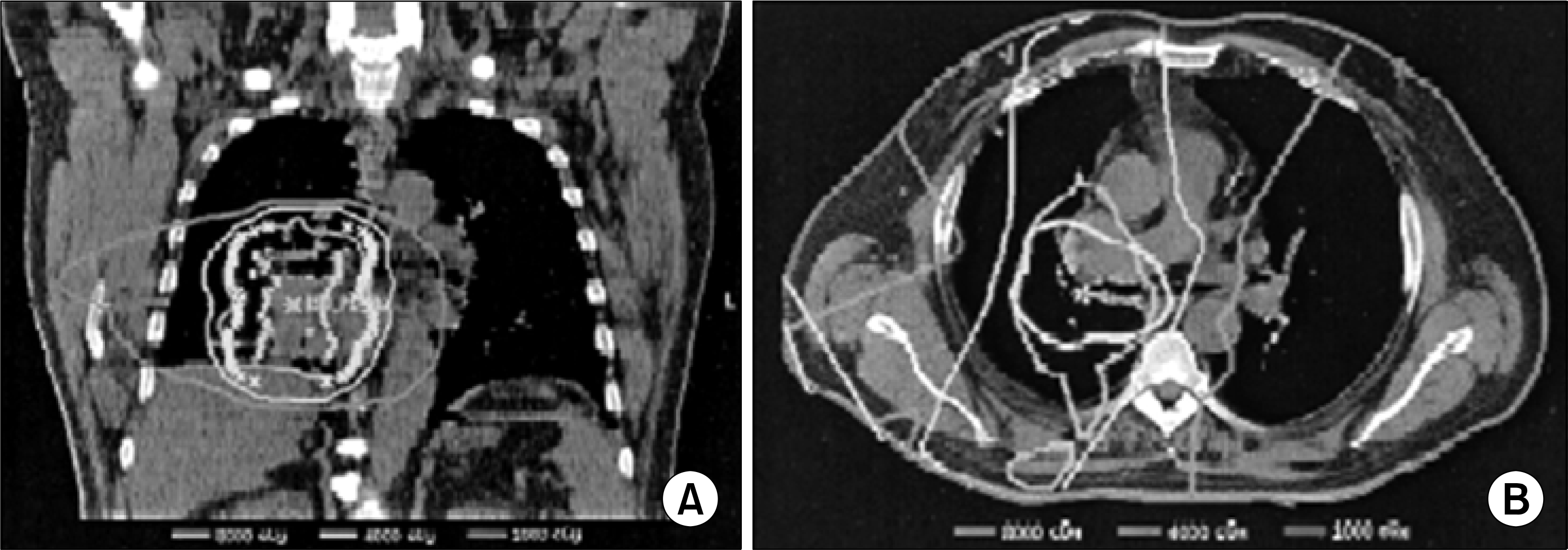
Fig. 2.
Compared 2-dimensional radiation treatment planning and 3-dimensiᄋnal radiation treatment planning.
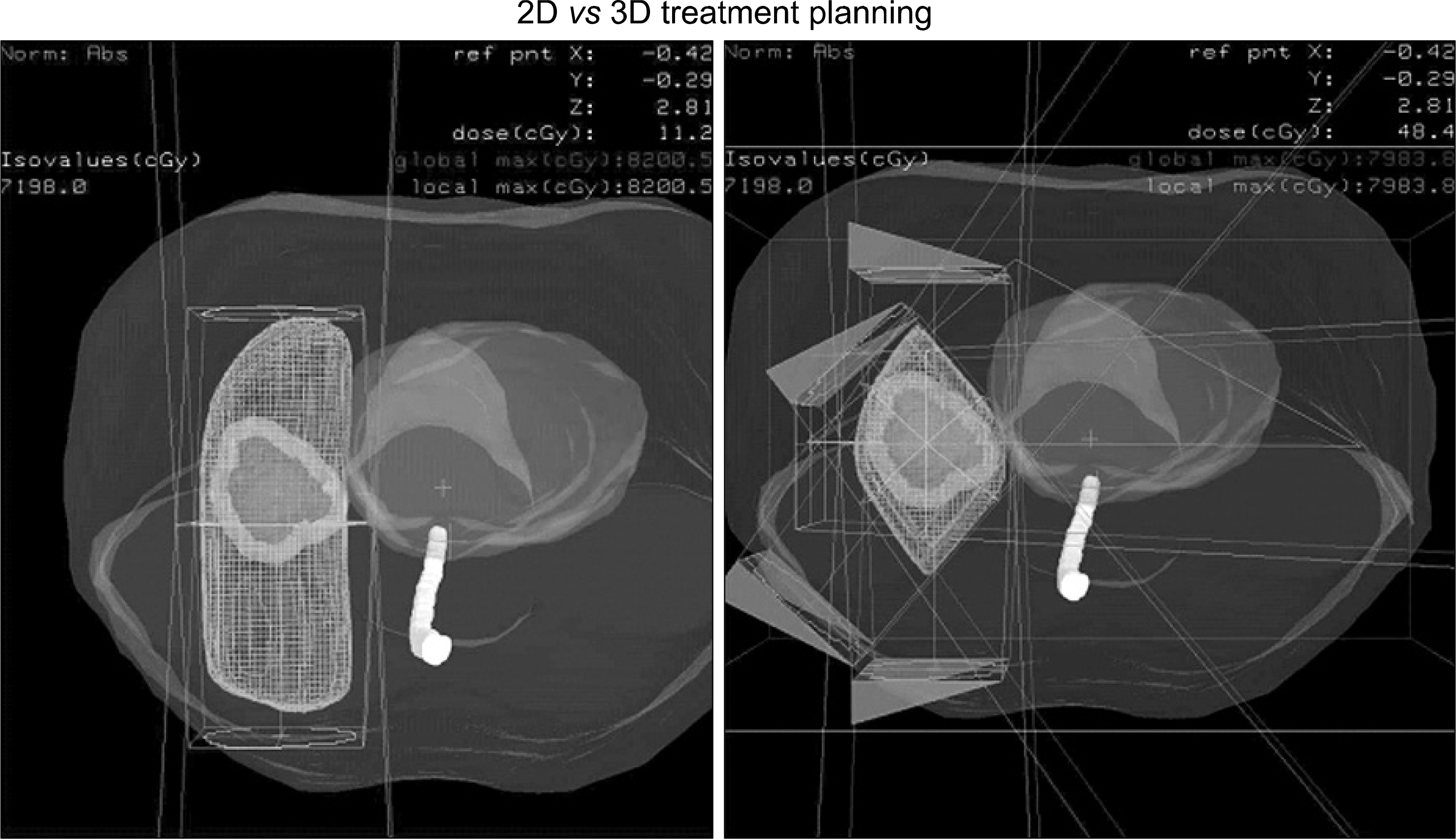
Table 1.
Multicenter Phase III Randomized Controlled Trials of Altered Fractionated Radiotherapy
Table 2.
Multicenter Phase III Randomized Controlled Trials Comparing Sequential Chemoradiation vs. Radiation Alone
Table 3.
Multicerrter Phase III Randomized Controlled Trials Comparing Concurrent With Sequential Chemoradiotherapy
Table 4.
Phase 11 Trials Comparing Induction or Consolidation Chemotherapy With Concurrent Chemoradiotherapy
Table 5.
Selected Chemotherapy Schemes in Multicenter Trials Studying Chemoradiation




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download