Dear Editor:
Imatinib mesylate (Gleevec; Novartis AG, Basel, Switzerland), a selective tyrosine receptor kinase inhibitor, is increasingly used for treating chronic myeloid leukemia, Philadelphia chromosome-positive acute lymphoblastic leukemia, and high-grade gastrointestinal stromal tumors (GISTs)1. Several cases of cutaneous reactions after imatinib use have been reported1. We report a case of EM after imatinib administration for the treatment of a GIST.
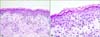
A 66-year-old woman was referred for pruritus from the department of oncology. She received a diagnosis of a GIST, for which she received adjuvant imatinib therapy after gastric wedge resection. She noticed a pruritic rash on her trunk after 5 weeks of 400 mg/day imatinib therapy. Physical examination revealed generalized variable-sized erythematous wheal-like patches with some targetoid lesions on the trunk, face, and extremities (Fig. 1). Immunoglobulin (Ig) G and IgM antibodies to the herpes simplex virus were not detected. A skin biopsy from the trunk revealed vacuolar degeneration, tagging of lymphocytes along the dermal-epidermal junction, and perivascular lymphocytic and some eosinophilic infiltrations in the upper dermis (Fig. 2A). Some dyskeratotic and necrotic keratinocytes were obvious in the epidermis (Fig. 2B); therefore, EM was diagnosed. As imatinib was the only medication administered to the patient, it was considered the most probable cause. Imatinib was discontinued, and oral steroid and antihistamine were prescribed. For 2 weeks, 30 mg/day steroid, tapered to 5 mg/day, was administered. One month after the discontinuation of imatinib therapy, the rash was fully cured. Imatinib treatment was restarted at a lower dose of 100 mg/day without steroids; no skin lesion developed for 2 months. However, when the dose was increased to 200 mg/day without oral steroids, a similar rash developed. The patient could continue imatinib therapy with a gradual dose escalation from 100 to 200 mg/day with concomitant 5 mg/day oral steroids. No additional skin lesions were detected during 5 months of follow-up.
It is estimated that approximately 7%~21% of patients treated with imatinib experience variable degrees of skin eruptions2. There are a few reports of imatinib-induced EM. Park et al.3 reviewed patients with cutaneous eruptions after imatinib therapy, and 2 of 10 patients (20%) had EM-like drug eruptions. The cutaneous adverse effects of imatinib are dose dependent and seemingly related to a pharmacological effect of the drug12. The most common skin reactions are maculopapular rashes12, and these are spontaneously relieved with a minimal dose of antihistamine or topical steroids. Several uncommon or severe reactions such as Stevens-Johnson syndrome have also been reported; however, they seem to be an idiosyncratic adverse reaction of hypersensitivity4. These reactions require the concomitant use of oral steroids, and immediate discontinuation of imatinib therapy is imperative45.
Here, we observed a case of imatinib-induced EM. We noted a dose-dependent relation; thus, we consider that imatinib-induced EM is related to a pharmacological effect of imatinib. The skin lesion subsided and did not recur only with the concomitant use of low-dose oral steroids. As imatinib is a very effective therapeutic agent and alternative treatment options are limited, recognition of adverse cutaneous reactions after imatinib therapy and the appropriate management required is helpful.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download