Dear Editor:
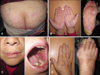
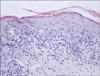
A 61-year-old Chinese woman was diagnosed with chronic myeloid leukemia (CML) in May 2012. One week later, imatinib mesylate treatment was started at a daily dose of 400 mg. Complete resolution of the CML was achieved within 12 weeks. However, 8 weeks after the imatinib treatment was initiated, the patient noticed several pruritic, violaceous, flat-topped papules on her waist and buttocks. The lesions were gradually increasing in number and size, and they progressed to her scalp and lower extremities. The physical examination revealed violaceous macules and flat papules with mild scaling on her trunk, buttocks, and lower extremities. No Wickham's striae were noted. There were large dark erythematous patches covered with thick, silvery lamellated scaling on her palmaris et plantaris. Her fingernails and toenails were thickened and appeared yellow-brown in color with a keratotic material accumulating under them. Erosive lesions and hemorrhagic crusts were observed on the lips, and reticular white striations were observed on the buccal mucosa (Fig. 1A~D). The histopathological examination of the abdomen revealed focal parakeratosis, liquefied degeneration of the basal cells, and exocytosis of mononuclear cells into the epidermis. There was a band-like infiltrate in the superficial dermis, which predominantly consisted of lymphocytes, sparse eosinophils, histocytes, and pigmentophages (Fig. 2). In addition, perivascular and periadnexal infiltrates were observed in the dermis. The results of routine laboratory tests were basically normal. The imatinib mesylate treatment was continued, and oral mizolastine and topical steroids were administered simultaneously. After 2 weeks, the small partial lesions on the trunk, buttocks, and lower extremities improved, but the medications did not help the palmoplantar lesions, mucous membrane lesions, or nail abnormalities. The patient is currently undergoing follow-up care.
Imatinib mesylate is the first molecular-targeted drug that is effective and tolerant in patients with CML or gastrointestinal stromal tumors. Side effects due to imatinib mesylate treatment are common, with an incidence rate of approximately 31%~44%1. However, a lichenoid reaction due to imatinib mesylate is rare, and only about 10 cases have been reported in the medical literature2,3; no cases have been reported in China.
A lichenoid reaction due to imatinib mesylate treatment has the following features2: 1) a long latency period (1~6 months); 2) the lesions mainly affect the patient's trunk and extremities, but sometimes the face, neck, palmaris et plantaris, and whole body can also be affected; 3) the lesions are limited to the skin, mucosa, or both; 4) nail dysplasia occurs in a few cases; and 5) the lesions are associated with a dose of imatinib mesylate (≥400 mg daily). Thus, the lichenoid reaction due to imatinib mesylate treatment is related to pharmacological effects rather than to a hypersensitivity reaction4.
The characteristic features of our current case included violaceous, flat-topped papules; palmoplantar changes; mucous membrane lesions; and nail abnormalities. This is the second reported case of such a reaction. The first reported case was a 31-year-old male who had a good response to oral prednisolone despite ongoing treatment with imatinib5




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download