Dear Editor:
Cutaneous squamous cell carcinoma (cSCC) is the 2nd most common non-melanomatous skin cancer after basal cell carcinoma (BCC). Although it is less common than BCC, it is associated with substantial risk of metastasis, and thus, accounts for the majority of deaths caused by non-melanomatous skin cancer. At present, it is widely accepted that in most cases cSCC evolves from precursor lesions, such as actinic keratosis and Bowen disease1. CD10 is a 90 to 110 kDa cell surface zinc-dependent metalloprotease which regulates biological activities of peptides substrates by reducing its local concentrations available for receptor bindings and signal transduction2. A recent study proved its association with tumor progression through comparing the CD10 expression between benign melanocytic nevi and malignant melanoma, and has concluded that it was a significant and independent prognostic factor in malignant melanoma3. Until now, it has not been identified whether CD10 expression in cancer cells could be associated with tumor progression in the cSCC. Therefore, in the present study, we compared the CD10 expression in actinic keratosis, Bowenoid actinic keratosis, Bowen disease, and cSCC, using tissue microarray (TMA) to identify whether CD10 could be a marker for malignant transformation of keratinocytes.
The cases of actinic keratosis, Bowenoid actinic keratosis, Bowen disease, and cSCC, as diagnosed in the Department of Dermatology, Gachon University School of Medicine (Incheon, Korea), between the years of 1999 and 2004, were collected. All diagnostic specimens were submitted either from punch (68 cases) or excisional biopsy (42 cases). The slides of all cases were re-reviewed by two dermatologists and one pathologist to confirm the diagnosis. A total of 25 samples of the cSCC were obtained and 28, 28, and 29 cases of actinic keratosis, Bowenoid actinic keratosis, and Bowen disease, respectively, were included for comparison. A representative 2.0-mm-diameter core biopsy was taken from one paraffin-embedded donor tissue block per case, and was subsequently arranged in new recipient paraffin blocks with a trephine (Quick-Ray; UNITMA, Seoul, Korea). In cases with variable histologic features, the predominant area was selected to construct TMA blocks. Serial sections from TMA blocks were subjected to immunohisto-chemistry (IHC). An adequate case was defined as a tumor occupying more than 50% of the core area. The specimen from the cSCC and normal tissue were included in each assay as positive and negative controls. Immunostaining was performed with monoclonal antibody directed against CD10. Semiquantitative assessment of the CD10 IHC stain results was performed by one pathologist (P.S.H) who was unaware of the clinicopatholoigcal details. Only membranous staining was defined as positive. The IHC pattern was relatively homogeneous, and thus, the score was determined by the predominant intensity. The expression was scored on the basis of the intensity and proportion of positive cells. The intensity score was defined as follows: 0=no appreciable staining in the tumor cells, 1=faint/barely perceptible partial membrane staining, 2=weak to moderate staining of the entire membrane, and 3=strong staining of the entire membrane. The proportion score was defined as follows: 0=less than 5%, 1=from 5% to 25%, 2=from 26% to 50%, 3=from 51% to 75%, and 4=more than 75%. The total score was calculated by multiplying the intensity score and the proportion score, producing a total range of 0 to 12. For statistical analyses, scores of 0 to 3 were considered negative, and scores of 4 to 12 were considered positive. All statistical analyses were performed using the SPSS statistical software for Windows (version 12.0; SPSS Inc., Chicago, IL, USA). The differences in CD10 expression between actinic keratosis, Bowenoid actinic keratosis, Bowen disease, and squamous cell carcinoma were evaluated by the Mann-Whitney U test.
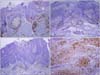
Twenty-eight cases of actinic keratosis, 28 cases of Bowenoid actinic keratosis, 29 cases of Bowen's disease, and 25 cases of SCC were included in this study. No specimens, other than cutaneous squamous cell carcinoma (cSCC), were immunostained with CD10 (Table 1, Fig. 1). Eight (32%) of 25 cases were positively immunostained for CD10 in cSCC. Certainly there was statistically significant difference of CD10 expression between cSCC and other lesions (p=0.000).
It is well-known that cSCC is the result of progression from the 'so-called' precancerous skin lesions, such as actinic keratosis, Bowenoid actinic keratosis, and Bowen disease. Therefore, demonstration of the role of CD10 expression in the cSCC, comparing the positivity of CD10 with precancerous conditions could be important to document the initiation and progression of cSCC. There have been two previous studies which dealt with CD10 immunostaining in cSCC and precursor conditions4,5, but each of those studies compared the positivity of stromal cells rather than tumor cells, and did not address the tumor cell immunoreactivity. In the results of the present study, CD10 expression was limited to the cases of cSCC, and no immunoreactivity was seen in the precursor conditions. CD10 expression was seen in 8 of 25 cSCC in the membrane of cancer cells, as well as stromal cells. CD10 expression was limited to the cSCC, which showed dermal invasion, and, in contrast no expression was identified in the precursor conditions which were characterized by lack of the dermal invasion. This phenomenon could be inferred as CD10 participating in the dermal invasion of cancer cells. Similar results were observed in studies of malignant melanoma. CD10 expression was stronger in tumor and stromal cells of metastatic melanoma than in primary melanoma2,3. In addition, CD10 expression was strongly associated with Breslow thickness and Clark level of malignant melanoma with the stronger expression of CD10 in the deeper invasion, than with superficial invasion of melanoma cells3,6. CD10 is a zinc-dependent metalloproteinase which participates in the breakdown of various proteins secreted into the intercellular space. Therefore, it could be postulated that CD10 destroys certain proteins which prevent the signal transduction of cellular migration and facilitate the dermal invasion of cancer cells.
In conclusion, the results from the present study suggested that CD10 expression is probably associated with tumor progression in cSCC. This study could provide further support for those who insist that the expression of CD10 protein is associated with tumor progression and poor prognosis in the cSCC. Further studies are needed to clarify the role of CD10 in cSCC.
This study was approved by the institutional review board (IRB number: Gilba2405).
Figures and Tables
References
2. Bilalovic N, Sandstad B, Golouh R, Nesland JM, Selak I, Torlakovic EE. CD10 protein expression in tumor and stromal cells of malignant melanoma is associated with tumor progression. Mod Pathol. 2004; 17:1251–1258.

3. Oba J, Nakahara T, Hayashida S, Kido M, Xie L, Takahara M, et al. Expression of CD10 predicts tumor progression and unfavorable prognosis in malignant melanoma. J Am Acad Dermatol. 2011; 65:1152–1160.

4. Takahara M, Chen S, Kido M, Takeuchi S, Uchi H, Tu Y, et al. Stromal CD10 expression, as well as increased dermal macrophages and decreased Langerhans cells, are associated with malignant transformation of keratinocytes. J Cutan Pathol. 2009; 36:668–674.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download