Introduction
Vertebrae are complex structures, consisting of a body, a vertebral arch, and various processes, such as the transverse, spinous, articular, accessory, and mammillary processes, which allow muscular or articular connections. The vertebral arch consists of right and left pedicles and laminae [7]. Each part of a vertebra consists of cortical and cancellous bones, which differ in density and architecture. Cortical bone has osteons, which provide cortical bone strength, but limited flexibility [23]. Cancellous bone is made up of trabeculae, which are avascular bone structures. The trabeculae are surrounded by adipose tissue and hematopoietic space that includes the bone marrow [23]. On computed tomography (CT) images, these differences in bone composition result in cortical bone appearing more compact and hyperattenuated than cancellous bone, which has a coarser structure and has a trabecular pattern. In general, cancellous bone is less dense than cortical bone and is more susceptible to early bone loss, which is affected by age, sex, hormones, body weight, and drugs [4614]. Various diseases can influence vertebral bone density. In particular, focal or multifocal changes in vertebral bone density can be identified with inflammatory diseases or tumor. In humans, differential diagnosis of focal sclerosis in vertebra are solitary osteoblastic metastasis, enostosis, compression fracture, pedicle sclerosis, and idiopathic [16]. Among those, solitary osteoblastic metastasis is mainly related with prostatic carcinoma, breast carcinoma, and lymphoma [13]. In dogs, vertebral metastasis can occur in carcinoma, particularly originating from the urinary tract or prostate. Vertebral tumors mainly show up as hypoattenuating areas on vertebral bone, caused by osteolysis; however, osteosclerosis is occasionally present on the vertebral bodies as hyperattenuated areas [20]. Solitary plasmacytomas, one type of plasma cell tumor originating from vertebrae, are described infrequently in dogs and are identified as focal radiolucent regions within a vertebral body or pedicle [17]. In discospondylitis, an inflammatory disease of intervertebral disks, the infection spreads from the disk to the vertebral endplate and, finally, to the vertebral body. Thus, vertebrae show hypoattenuation at the affected vertebral endplate and vertebral body due to irregular bone lysis [39]. Osteomyelitis also induces focal or multifocal osteolysis with or without osseous production in vertebrae. Therefore, focal change in vertebral bone density should be closely assessed to determine whether there are pathologic bone changes.
Widely used to investigate vertebral disorders, CT eliminates superimposed structures, enhances visualization of specific structures, and allows elucidation of anatomic relationships with surrounding structures through reconstruction on multiple anatomic planes [19]. In particular, CT can distinguish density differences of 0.5%, while conventional radiography can only detect a 10% difference in density [19]. Focal changes in bone density on CT images can provide early evidence of bone disease and can allow determination of the characteristics and extent of the bone changes. However, we have observed various focal hyperattenuation or hypoattenuation changes on vertebral CT images of healthy dogs that did not have pathologic bone disorders. The objective of this study was to describe the prevalence of focal vertebral density changes on CT images of clinically healthy dogs.
Materials and Methods
This descriptive study was performed on 20 dogs that underwent vertebral CT examination at the Chonnam National University Veterinary Teaching Hospital from 1 October 2011 to 31 May 2014. They were healthy, based on complete blood count, serum chemistry, and radiography results. In addition, the dogs had no history or clinical signs of vertebral or musculoskeletal diseases, neoplasia in any part of the body or metabolic or inflammatory diseases affecting bone density. Main purposes of their CT examination were to investigate lesions associated with ear canal, nasal cavity, salivary gland, subcutaneous tissue (such as foreign body), and others.
Vertebral CT images were achieved by using a 16-channel multi-detector CT (Siemens SOMATOM Emotion 16; Siemens, Germany) in sternal recumbency under general anesthesia. Anesthesia was induced with a combination of 1.5 mg/kg zolazepam/tiletamine (Zoletil; Virbac, France) and 0.03 mg/kg medetomidine (Domitor; Orion, Finland) intramuscularly, or by inhalation of isoflurane (Isoflurane; Rhodia Organique Fine, UK) with oxygen after induction with a combination of 0.5 mg/kg zolazepam/tiletamine and 0.01 mg/kg medetomidine according to the patient's condition. CT images were obtained at 110 to 130 kVp and 200 mAs and were reconstructed with a slice thickness of 1 to 2 mm.
All transverse CT images were processed on an INFINITT picture archive and communication system (PACS; INFINITT Healthcare, Korea) and evaluated, using a 450 Hounsfield unit (HU) window level and a 1,500 HU window width, by two examiners (J Choi and S Park), with the final results obtained by consensus. Focal vertebral changes showing hyper- or hypoattenuation were assessed at the spinous process, transverse process, and body of each vertebra and the number of changes (single or multiple), the location, and the density (hyperattenuation or hypoattenuation) were determined. Vertebral changes that were repeatedly seen on vertebral CT images of dogs were not included in this study, as they were considered to be related to the normal trabecular pattern. Bone density changes that were intermittently observed, without a consistent pattern, were defined as focal vertebral bone density changes.
Statistical analysis was performed by using statistical software (IBM SPSS Statistics ver. 21; IBM, USA). Fisher's exact test was used to investigate the association of gender and the incidence of focal vertebral bone changes. When distribution normality was met, Student's t-test was used to investigate the association of age and the incidence of focal vertebral bone changes. If normality conditions were not met, the Mann-Whiney U test was used to investigate the association between body weight and the incidence of focal vertebral bone changes. A p<0.05 was considered significant for all analyses.
Results
Twenty dogs of 10 different breeds were enrolled in this study. Most dogs were small breeds, such as Maltese (n = 8), Shih Tzu (n = 2), Yorkshire terrier (n = 2), mixed (n = 2), Pekingese (n = 1), poodle (n = 1), and miniature Schnauzer (n = 1); other breeds were Beagle (n = 1), Jindo (n = 1), and Shetland sheep dogs (n = 1). The mean body weight was 4.67 kg (range, 1.6–10.5 kg). There were 13 female (4 spayed female) and 7 male (2 male castrated) dogs. The mean age of all dogs was 6.05 years (range, 3 months to 13 years). Vertebral changes did not have a significant correlation with age (p=1.000), bodyweight (p=0.117), or gender (p=0.206).
The characteristics of the vertebral changes were assessed by examining cervical, thoracic, and lumbar regions. Because this study did not primarily aim to investigate vertebral morphologic variations, the vertebral CT images were not always scanned from cervical to lumbar regions in all 20 dogs. A total of 429 vertebral CT images were obtained: 63 images from 9 cervical regions, 247 images from 19 thoracic regions, and 119 images from 17 lumbar regions.
The vertebral body consists of outer hyperattenuating compact cortical bone and inner hypoattenuating cancellous bone with a coarse structure, regardless of the vertebral region. A normal trabecular pattern was more prominent in the thoracic and lumbar vertebrae than in the cervical vertebrae. Spinous and transverse processes were also made of cortical and cancellous bones; however, hypoattenuating cancellous bone was not prominently observed, particularly in the cervical vertebrae.
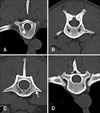
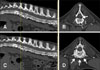
Vertebral CT images of 14 dogs intermittently showed hyper- or hypoattenuating changes in the vertebral bone. A total of 99 focal vertebral bone density changes were identified from the 429 vertebral CT images. The number of bone changes according to vertebral region varied, and one vertebra even showed 2 different changes. Focal vertebral bone changes were mainly found in thoracic vertebrae (73 lesions in 247 CT images, 29.6%), followed by cervical vertebrae (9 in 63, 14.3%), and lumbar vertebrae (10 in 119, 8.4%). The incidence of single and multiple changes were approximately equal, although solitary changes (51.5%) occurred slightly more often than multiple changes (48.5%). Most focal vertebral bone changes were hyperattenuating (86.9%) and only 13.1% were hypoattenuating. Among the hyperattenuating changes, multifocal changes (53.5%) were more common than single changes (46.5%) (Fig. 1). Most of the hypoattenuating changes occurred as single changes (92.3%). All focal vertebral bone changes were observed in the vertebral body, except for one single hyperattenuating change in a thoracic transverse process. Eight dogs, 40.0% of the 20 study dogs and 61.6% of the 13 dogs showing focal vertebral bone changes in thoracic vertebrae, had hyperattenuating changes at the 7th or 8th thoracic vertebra, including 11 multiple and 5 single changes.
Six dogs had spondylosis deformans, but the location of spondylosis deformans was not related to hyperattenuating changes (Fig. 2).
Discussion
In this study, many vertebral bone changes were identified in healthy dogs that did not have tumors or inflammatory vertebral and musculoskeletal diseases. Vertebral density changes are very important factors when investigating primary or secondary vertebral disease. Focal or multifocal vertebral lesions have been considered to be intrinsic vertebral pathologic changes when compared with diffusely increased or decreased vertebral density, which can be found in various endocrine and metabolic disorders. However, focal or multifocal changes in vertebral density were incidentally identified on vertebral CT images in healthy dogs, in the absence of accompanying general or focal pathologic conditions.
A vertebral bone density change that we repetitively identified in many dogs was hypoattenuating area presence in the vertebral body, transverse process, and spinous process. These changes were related to the trabecular pattern of the cancellous bone, which was coarser than the surrounding cortical bone density. Focal vertebral bone changes that we identified intermittently were distributed from the cervical to the lumbar regions, and were more prominent in thoracic vertebrae: a total of 29.6% of thoracic vertebrae, 14.3% of cervical vertebrae, and 8.4% of lumbar vertebrae were involved. The high occurrence of vertebral bone density changes in thoracic vertebrae may be related to the stability of the vertebral bone. The moment of resistance of the vertebral bone represents the inner forces or strength of the vertebral columns, which offer resistance to bending [24]. The moment of resistance of vertebrae is affected by the rib, spinous process, transverse process, articular process, muscle, ligament, and other structures. Thoracic vertebrae have the lowest moment of resistance in dogs, as these vertebrae are stabilized by the surrounding structures [24]. In contrast, the moment of resistance value is highest in lumbar vertebrae, due to the lack of a dependable surrounding structure. The moment of resistance is associated with bone mineral density. In a previous study, the bone mineral density from the 11th thoracic vertebra to the 3rd lumbar vertebra was statistically correlated with the moment of resistance [24]. Bone mineral densities of the 2nd and 3rd lumbar vertebrae were slightly decreased, while the moments of resistance increased at these regions. In our study, focal hyperattenuating vertebral bone changes were observed in the 7th and 8th thoracic vertebrae in about 40% of the 20 dogs, and 61.6% of 13 dogs showing focal vertebral changes in the thoracic vertebrae. Considering that the moment of resistance of the thoracic vertebra are reported to be lowest at the 6th, 7th, and 8th thoracic vertebrae, the incidence of focal vertebral bone changes may be related to the stability of vertebral bone, although the moment of resistance of these vertebrae was not determined in the present study [24].
Most focal vertebral bone changes were hyperattenuating, regardless of whether these occurred in isolation or whether there were multiple changes. Hyperattenuating changes in vertebrae are observed mainly in the presence of tumors or inflammatory diseases. Primary vertebral tumors are mostly accompanied by proliferative as well as osteolytic changes. However, proliferative bone changes in vertebral bone tumors usually occur at the exterior of the vertebral body, and not as a focal increase of bone density within the vertebral body. Moreover, vertebral tumors are mostly accompanied by osteolysis on the vertebral pedicle and body [5]. In human, focal sclerosis in a vertebra can be seen in patients with metastatic tumors [13]. In 80% to 90% of prostatic carcinoma, vertebral metastasis is osteoblastic, and 10% of breast carcinoma to vertebral metastasis has been reported as solitary osteoblastic lesion. Vertebrae are also the predilection site for metastatic carcinoma in dogs. On histopathologic examination, metastatic carcinoma mainly exhibits an intertrabecular growth in vertebrae and only progresses to osteolysis and proliferative changes in an advanced stage [8]. New bone, in vertebral metastasis, is generated on the ventral aspect of the vertebral body and in paravertebral soft tissue; thus, solitary hyperattenuating lesions described in the present study have different features from vertebral metastasis. The hyperattenuating appearance of a vertebra in inflammatory disease can be seen as a secondary change, with infectious bone destruction or marked bone formation occurring on the outer surface in noninfectious inflammatory disease. Enostosis is an infrequently identified focal sclerotic lesion in vertebrae and is composed of compact lamellar bone and Haversian systems that mix with the adjacent spongiosa [12]. Enostosis ranges from 1 to 20 mm in diameter, or forms giant bone islands when the enostosis is larger than 2 cm [11]. This is considered to be a developmental error in the processing of endochondral ossification [2]. In humans, enostosis appears as round and ovoid foci of osteosclerosis in cancellous bone, with homogeneous density on radiographs [2]. Moreover, on CT images, enostosis has characteristic brush borders, with bony spicules radiating from the periphery of the lesion and intermingling with the surrounding trabeculae. In our study, 13 vertebral changes, observed in 1 cervical vertebra, 9 thoracic vertebrae, and 2 lumbar vertebrae, appeared as hyperattenuating areas within the vertebral trabecular bone on CT images. Most of these occurred in isolation, except for 1 which accompanied multiple changes. There are no previous reports about vertebral bone enostosis in veterinary medicine; however, considering the characteristic appearances of enostosis in human literature, none of the hyperattenuating changes observed in the present study were suspected to represent enostosis. In human, idiopathic focal sclerosis in a vertebra can be suspected when there is no evidence of underlying pathology, and in which there is no increased radionuclide uptake on bone scintigraphy [15]. Considering radiographic characteristics of idiopathic vertebral sclerosis, including the predilection of sclerosis on the anterior of the vertebral body adjacent to the end-plate and disc space narrowing, hyperattenuating lesion in the present study could not be determined as idiopathic focal sclerosis.
Solitary or multifocal hypoattenuating vertebral bone changes can be caused by tumors or inflammatory diseases, as occurs with hyperattenuating changes. Discospondylitis, a vertebral inflammatory disease, causes focal hypoattenuating lesions in the vertebral body, which center on intervertebral disks and spread into the endplate and vertebral [20]. The diagnosis of discospondylitis can be performed based on typical radiographic findings; no vertebral lesions consistent with discospondylitis were observed in this study. Metastatic vertebral tumors can present as multifocal changes on CT images. Sole occurrence of osteolysis on vertebral bone was more prevalent than osteolysis accompanying proliferation [1]. CT can detect tumor metastasis that precedes more obvious osteolysis by increased attenuation of bone marrow associated with a change of normal bone marrow fat into tumor [18]. Bone marrow density of the vertebrae in this study was not diffusely increased, and primary tumor suspicious lesions adjacent to vertebral changes were not detected in this study.
Hypoattenuating vertebral bone changes may be related to some infrequent diseases, such as pneumatocysts, hydatid cysts, and others. In pneumatocysts, gas originating from the intervertebral disc enters the vertebral body through a space in the osteocartilaginous epiphyseal plate and accumulates within it [21]. Thus, pneumatocyst can be diagnosed when air-equivalent attenuation of the lesion is located close to the degenerated vertebral endplates and gas in the degenerated intervertebral discs is observed on CT images [21]. One lesion among a total of 13 hypoattenuating vertebral bone changes appeared similar to a pneumatocyst, but the HU value of the lesion was +18, which was higher than that of gas, and there were no degenerative changes in the adjacent vertebral endplates. Hydatid cysts form in any organ and tissue due to Echinococcus granulosus but occur more frequently in the vascularized area of bones [10]. Hydatid cysts appear as a bone cyst, having a round or oval osteolytic lesion with a sclerotic margin [22]. Although the cyst was not examined through fine needle aspiration in this study, there were no round or oval osteolytic lesions with sclerotic margins observed. Considering the characteristics of pneumatocysts and hydatid cysts, none of our hypoattenuating changes were suspected as being pathologic lesions.
There are some limitations to this study. The designation of normal status in our dogs was not confirmed by histological examination of each lesion. Therefore, pathological changes in the vertebral bone of some dogs cannot be completely ruled out. However, this study was focused on focal vertebral changes, rather than on overall bone density, and we tentatively ruled out bone tumors and inflammation based on laboratory data, radiographs, and CT images.
In conclusion, focal vertebral bone changes were observed in many vertebrae, particularly in thoracic vertebrae, as hyperattenuating areas. Focal hyperattenuating vertebral bone changes may be related to vertebral bone stability, but the reason for many of the vertebral bone changes could not be explained by pathological conditions. The results of this study indicate that focal changes in vertebral bone density can be commonly identified on vertebral CT images in healthy dogs.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download