Introduction
Ectonucleotide pyrophosphatase/phosphodiestrase 2 (Enpp2), also known as autotaxin (ATX), is a 125-kDa secreted glycoprotein that is commonly detected in various biological fluids including in blood, synovial fluid, and cerebrospinal fluid. Enpp2 was originally isolated from the human melanoma cell line A2058 as an autocrine motility stimulation factor [8,12,27]. Enpp2 is thought to be a plasma enzyme because it is a member of the ectonucleotide pyrophosphatase and phosphodiesterase (NPP) family of ectoenzymes. NPP1 (PC-1) and NPP3 (gp130RB13-6, PD-Iβ, B10) share some characteristics with Enpp2, and are grouped together in the Enpp family [15,20,33]. However, only Enpp2 has lysophospholipase D (lysoPLD) activity, which has been observed in the microsomes of animals and produces the signaling molecule lysophosphatidic acid (LPA) from lysophosphatidylcholine (LPC) [36,39]. LPC is a major lysophospholipid and formed from phosphatidylcholine by phospholipase A2 [42]. It was recently reported that Enpp2 knock-out mice die on embryonic day 9.5 because Enpp2 plays a critical role in blood vessel formation in the yolk sac [35]. In addition, Enpp2 is able to activate cell motility through LPA production.
Enpp2 has a catalytic site that has been shown to regulate cell motility [22]. When a threonine residue (Thr210) in the catalytic site is replaced with an alanine, Enpp2 loses the ability to stimulate cell motility [14]. LPA produced by Enpp2 stimulates the motility of various types of cells [13,23,29]. Furthermore, this factor facilitates cell proliferation and migration during various biological processes including neurite retraction, platelet aggregation, smooth muscle contraction, actin stress formation, and cytokine and chemokine secretion by activating G protein-coupled receptors (GPCRs) to elicit multiple cellular responses [18,26,37].
LPA can be found in many biological fluids, including serum and follicular fluids [31], and acts through at least six GPCRs which are divided into two subgroups. One subgroup consists of receptors in the endothelial differentiation gene (EDG) family and the other includes receptors in the P2Y receptor family. LPA receptors (LPAR)1/EDG2, LPAR2/EDG4 and LPAR3/EDG7 belong to the EDG family receptors, and LPAR4/GPCR23, LPAR5/GPCR92 and LPAR6/P2Y5 are members of the P2Y family receptors. Enpp2 can carry out multiple functions through these LPARs [1,2,29,40]. In particular, LPAR3 has been shown to affect the female reproductive system. A previous study demonstrated that LPAR3-deficient mice had significantly reduced litter sizes and reduced rates of embryo implantation, indicating that LPAR3 can delay implantation and alter embryo spacing [41]. LPAR3 is therefore thought to play a critical role in the mammalian female reproductive system. Another recent report showed that mouse uterine LPAR3 mRNA expression was altered during early pregnancy and throughout the estrous cycle [41]. In addition, LPAR3 is regulated by progesterone and estrogen in the mouse uterus, and is regulated during early pregnancy at least in part by transcriptional regulation and subsequent LPAR3 signaling [16].
The biological effects of estrogen (E2) and progesterone (P4) are mediated through estrogen receptors (ERs) and progesterone receptors (PRs), respectively, to regulate growth and differentiation in the human body [5,17]. ER has two different forms (ERα and ERβ) and is a member of the nuclear hormone family of intracellular receptor [7,24]. ER serves as ligand-activated transcription factor. PR also has two different forms (PR-A and PR-B) in humans which are members of an important superfamily of transcription regulatory factors [38].
Multiple analyses have identified numerous estrogen-responsive genes like Enpp2, Igf2, and Igfbp2 in rat hippocampus [34]. However, little is known about the role of Enpp2 in the uterus during the estrous cycle and its regulation in this tissue is not well understood. Lysophosphatidic acid receptor 3 (LPAR3) is reportedly expressed in the uterus during the estrous cycle [41]. The expression and regulation of Enpp2, which catalyzes the synthesis of LPA, has not been studied in rats. Therefore, we examined the expression of Enpp2 in rat uterus during the estrous cycle. We also investigated the effects of the steroid hormones E2 and P4, along with receptor ER and PR antagonists, on the expression and regulation of uterine Enpp2.
Materials and Methods
Materials
E2 (17β-estradiol), P4 (progesterone), and mifepristone (RU486) were obtained from Sigma-Aldrich (USA). ICI 182780 was purchased from Tocris (UK).
Animal treatment
Mature (14 weeks old) and immature (14 days old) female Sprague-Dawley rats were obtained from Koatech (Korea). All animals were housed in polycarbonate cages and acclimatized to an environmentally controlled room (temperature: 23 ± 2℃, relative humidity: 50 ± 10%, frequent ventilation, and a 12-h light-dark cycle) before use. The mature rats were monitored for 14 days and daily vaginal smears were obtained and examined using a low-power light microscope. After this period of observation, the rats (n = 12) were separated according to estrous cycle stage: proestrus, estrus, and diestrus.
For hormone and antagonist treatments, immature female rats (n = 30) with matched body weight were divided into six groups (five rats each a group). The steroid hormones E2 (1.2 µg) or/and P4 (120 µg) were dissolved in 50 µL of 10% ethanol (Sigma, USA) for an immature rat. And two steroid solutions were administered to the rats by subcutaneous injections. The animals were treated with E2 (40 µg/kg BW), P4 (4 mg/kg BW), or E2 and P4 for 3 days, and were then euthanized 12 h after the final injection.
To determine the effects of E2, 10 immature rats were divided into two groups that were treated daily for 3 days with subcutaneous injections of the estrogen receptor antagonist ICI 182780 (10 mg/kg BW) 30 min prior to injection with either E2 (positive control, n = 5, 40 µg/kg BW) or ethanol (negative control, n = 5). To measure the effects of P4, 10 immature rats were divided into two groups that were treated daily for 3 days with subcutaneous injections of the progesterone antagonist RU486 (10 mg/kg BW) 30 min prior to injection with either P4 (positive control, n = 5, 4 mg/kg BW) or ethanol (negative control, n = 5). The Ethics Committee of Chungbuk National University (Korea) approved all experimental procedures involving animals.
Total RNA extraction and semi-quantitative PCR
The rats were euthanized under deep ether anesthesia, and uteri were rapidly excised and washed in cold, sterile 0.9% NaCl. Total RNA extraction from uteri with TRIzol reagent (Invitrogen, USA) is a common method of total RNA extraction according to the criteria of [10], and the concentration of RNA was set by pure, concentrated of RNA to an optical density of 1.0 at 260 nm using a Perkin Elmer (MBA2000; USA) Spectrophotometer. The concentration of stock solutions were then calculated and used for all subsequent dilutions. Total RNA (1 µg) was then reverse transcribed to first-strand complementary DNA (cDNA) using moloney murine leukemia virus reverse-transcriptase (iNtRON Bio, Korea) and random primers (9-mers; TaKaRa Bio, Japan). Enpp2 cDNA was amplified in a 20 µg PCR reaction containing 0.5 U Taq polymerase (iNtRON Bio, Korea), 1.5 mM MgCl2, 2 mM dNTP, and 100 pmol of the specific primers. The oligonucleotide primers used for producing Enpp2 cDNA were 5'-AGC TGC CTG GAC TTC ACT CA-3' (sense) and 5'-GCA GGT ATG TCT TGA GGG TCA-3' (antisense). PCR reactions were carried out with denaturation at 95℃ for 30 sec, annealing at 58℃ for 30 sec, and extension at 72℃ for 30 sec. The 1A gene was used as an internal control to normalize total cDNA; this is the cytochrome c oxidase subunit 1 which is a key enzyme for aerobic metabolism. The primers for 1A were 5'-CCA GGG TTT GGA ATT ATT TC-3' (sense) and 5'-GAA GAT AAA CCC TAA GGC TC-3' (antisense). Enpp2 and 1A genes were quantified by performing 30 and 25 cycles, respectively. PCR products (8 µL) were separated on a 2.3% agarose gel and photographed using a Gel Doc EQ (Bio-Rad, USA).
Western blot analysis
Uteri were rapidly excised and washed in cold, sterile 0.9% NaCl. Soluble proteins were extracted using Proprep (iNtRON, Korea) according to the manufacturer's instructions. Sixty µg of total protein per lane was separated by SDS-PAGE on a 7.5% gel and then transferred to polyvinylidene fluoride transfer membranes (Perkin Elmer, USA) with a TransBlot Cell (TE-22; Hoefer, USA) according to the manufacturer's protocol. The blots were blocked in Tris-buffered saline with Tween 20 (TBS-T) containing 5% skim milk for 2 h at room temperature and then incubated with primary antibodies against Enpp2 (rabbit polyclonal, 1 : 500; Cayman Chemicals, USA) or beta-actin (mouse monoclonal, 1 : 4,000; Santa Cruz Biotech, USA). After washing in TBS-T (Tris-buffered saline with containing 5% Tween20) buffer, the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (anti-rabbit, 1 : 2,500, Santa Cruz Biotech, USA; or anti-mouse, 1 : 5,000, Santa Cruz Biotech, USA) for 2 h at room temperature. After washing using the 10 mL of TBS-T, antibody binding was visualized by incubating the blots in enhanced chemiluminescence chemiluminescence reagent (Amersham Biosciences, UK) and subsequent exposure to Biomax Light film (Kodak, USA) for periods ranging from 1 to 5 min. Signal specificity was confirmed by incubating the blots without either primary antibody. All bands were normalized to the β-actin immunoreactive bands on the same membrane after stripping (stripping solution is composed with SDS (10 g) and Tris (3.785 g). The density of each band was measured with ImageJ software (NIH, USA). Density measurements for each band were performed with NIH ImageJ software. Background samples from an area near each lane were subtracted from each band to obtain mean band density. Data were analyzed for significant differences by one-way ANOVA (Prism 4 Graph Pad; Graph Pad Software, USA).
Immunohistochemical staining
The localization of Enpp2 protein was determined using immunohistochemistry. Uteri were embedded in paraffin and then sectioned. Sections (5-µm thick) were then deparaffinized in xylene and hydrated in decreasing concentrations of ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in TBS-T for 30 min. Nonspecific reactions were blocked by incubating the sections in 10% normal goat serum (Vector, USA) for 2 h at room temperature. The sections were subsequently incubated overnight at 4℃ with a polyclonal rabbit antibody (1 : 500, Cayman Chemicals, USA) against Enpp2 dissolved in 10% normal goat serum. After washing with TBS-T, the sections were incubated with biotinylated secondary antibody (anti-rabbit IgG, 1 : 1,000; Vector Laboratories, USA) for 1 h at 37℃ and further incubated with ABC-Elite (Vector, USA) for 30 min at 37℃. Diaminobenzidine (Sigma, USA) was used as a chromogen, and the sections were counterstained with hematoxylin before being mounted in mounting solution (Cytoseal-60; Richard Allen Scientific Kalamazoo, USA).
Statistical analysis
Data were analyzed using a nonparametric one-way ANOVA using Tukey's test followed by Dunnett's test for multiple comparisons to the negative controls. Data were ranked according to these tests. All statistical analyses were performed using SPSS for Windows (SPSS, USA). p-values < 0.05 were considered statistically significant.
Results
Patterns of uterine Enpp2 mRNA and protein expression throughout the estrous cycle
To investigate expression of Enpp2 in rat uterus during the estrous cycle, rat uteri from each stage of the estrous cycle were classified according to vaginal cell morphology. Total RNA was extracted and RT-PCR was carried out using specific primers. The level of Enpp2 mRNA was significantly increased during diestrus compared to other stages of the cycle and was decreased during estrus (Fig. 1). Western blot analysis was performed to measure Enpp2 protein expression in rat endometrium throughout the estrous cycle. Enpp2 protein was highly expressed during diestrus, but the expression was reduced during proestrus and estrus (Fig. 2). These results indicate that the expression of uterine Enpp2 mRNA and protein are tightly regulated throughout the estrous cycle and may be influenced by steroid hormone fluctuations during this cycle.
Effects of the sex steroid hormones E2 and P4 on Enpp2
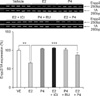
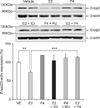
To investigate the effects of E2 and P4 on Enpp2 expression, immature rats were injected daily with E2 and/or P4 for 3 days. Uterine expression of Enpp2 mRNA and protein was significantly decreased by E2 compared to the vehicle (Figs. 3 and 4). To investigate the ER-mediated effects of E2 on uterine Enpp2 transcription, ICI 182780, an ER antagonist, was injected into immature rats 30 min prior to E2 treatment. Pretreatment with the ER antagonist completely reversed the E2-induced reduction of Enpp2 mRNA and protein expression (Figs. 3 and 4). This finding suggests that ERs are involved in E2-mediated regulation of Enpp2 expression in the uterus.
Localization of Enpp2 in rat uterus
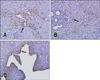
To examine the localized expression of Enpp2 protein in the uterus, we performed immunohistochemistry using an anti-Enpp2 antibody on tissue sections obtained during different stages of the estrous cycle (proestrus, estrus, and diestrus). Enpp2 protein was found in stromal cells during the proestrus and estrous stages. Enpp2 protein expression was also observed in endometrial luminal epithelial cells and the endometrial glands during diestrus (Fig. 5).
Discussion
Enpp2 has lysophospholipase D activity which enables it to catalyze the hydrolysis of LPC phosphodiester bonds and allow lysoPLD to generate the signaling molecule LPA from LPC [21,28,36]. Enpp2 has also been shown to act through LPAR. A recent report described uterine expression of LPAR3 during the estrous cycle, but the expression and regulation of Enpp2, an enzyme that has a major role in LPA production, has not been studied in rats [41]. Previous studies have also confirmed that the expression of LPAR3 mouse uterine is altered by estrogen and progesterone [16,19,41]. In addition, expression of LPAR3 plays a critical role in embryo implantation and early pregnancy [25]. Enpp2 plays a major role in LPA synthesis, and so we hypothesized that Enpp2 is also affected by sex hormones during the estrous cycle. In our study, we observed the highest expression of Enpp2 mRNA at diestrus stage. During diestrus stage, E2 and P4 levels are at the lowest of the during estrus cycle [11,32]. These results indicate that the level of Enpp2 expression was may be involved with sex steroids (E2, P4) of the rat during estrus cycle.
To determine the effects of these hormones on uterine Enpp2 transcription, immature rats were injected daily with E2 and/or P4. E2-treated rats showed lower uterine expression of Enpp2 mRNA and protein. These results imply that E2 may be an inhibitory regulator of uterine Enpp2 transcription in rats. Although these findings suggest that expression of the Enpp2 gene is reduced in response to estrogen in rat uterus, the relation between Enpp2 and steroid hormone metabolism is unknown. In this study, we observed ER and PR-mediated Enpp2 expression following ER and PR antagonist treatment. However, we need to elucidate the detailed mechanism(s) of ER and PR-mediated Enpp2 expression in further studies.
A published report showed that the expression of Enpp2 is estrogen-responsive and that its expression is cell type-specific [34]. A recent study examined the expression of ovine uterine Enpp2 throughout the estrous cycle and during pregnancy [25]. However, it was found that Enpp2 mRNA levels are not significantly different on days 12 to 15 of pregnancy compared with those of non-pregnant females [25]. We postulate that the expression of Enpp2 varies according to cell type in the reproductive system as a result of this hormonal regulation.
Enpp2 (ATX) is a known factor that stimulates tumor cell motility. We believe that Enpp2 also plays a role in regulating the proliferation of uterine cells. Using immunohistochemistry, we analyzed the localization of Enpp2 protein during the estrous cycle and the effects of sex steroid hormones on Enpp2 expression. Enpp2 expression varied throughout the estrous cycle. The result, during the proestrus and estrus stages, Enpp2 was detected primarily in stromal cells. However, this protein was detected in luminal epithelial cells during diestrus.
Most previous studies of this protein have focused on its ability to regulate the proliferation of uterine myoma cells [6]. LPA has been shown to stimulate stress fiber formation and to have a mitogenic effect on the human myometrium [6]. It was recently reported that uterine leiomyoma cell proliferation is regulated by phospholipase D and Enpp2 [6]. According to previously reported that LPA induces the expression of IL-8 through LPAR1 [40]. LPAR1 is expressed during the proliferative and secretory phases in human endometrial stromal cells. LPA is a critical mediator of the MAPK/p38 and NF-κB signaling pathways [9]. Thus, we postulate that LPA also mediates reproductive function because Enpp2 plays a critical role in the reproductive system by producing LPA. LPA can be found in epithelial cells, stromal cells, and blood vessels [31]. Thus, we believe that LPA may act as an autocrine and paracrine mediator of epithelial cell-to-epithelial cell and epithelial cell-to-stromal cell communication [31].
The endometrium undergoes structural modification and changes during the estrous cycle in rodents [4]. E2 and P4 can also mediate changes in the structure and function of the uterus and control the estrous cycle [3,30]. Endometrial proliferation occurs during the proestrous and estrous stage, and is degenerated during diestrus by steroid hormones. In this study, we observed that migration of ENPP2-induced stromal cells from basolateral side to the cytoplasm of endometrium during proestrus, estrus and diestrus cycle. It can be assumed that Enpp2 expression may be involved in endometrial cell proliferation and migrate from the basolateral side to endometrial layer in the stromal cells of rat uterus.
Although we were not able to confirm the precise localization of LPA expression, we believe that it may be similar to that of Enpp2 because LPA is synthesized by Enpp2. We do not know why Enpp2 expression is inhibited by estrogen, but the results from other studies indicate that Enpp2 expression differs according to cell type and species [34].
In summary, we examined the expression of Enpp2 mRNA and protein in rat uterus, and showed for the first time that this expression fluctuates during the estrous cycle. In addition, the uterine expression of Enpp2 mRNA and protein appears to be regulated by E2, suggesting that uterine Enpp2 may play an important role in the female rat reproductive system. As referred to earlier, new research will be required to obtain further insights into the detailed mechanism(s) of ER and PR-mediated Enpp2 expression. We were expected to interesting results that LPA and Enpp2 should be studied together in this model.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download