Abstract
Soft tissue metastasis clinically presenting as either painless subcutaneous or painful intramuscular nodules is extremely rare and may lead to an errant clinical suspicion of sarcoma. In general, most soft tissue metastasis comes from lung carcinoma; however, to date, there have been no reports of a posterior thigh mass just beneath the skin metastasizing from breast cancer. Here, we report a case of distant soft tissue metastasis presenting as a painless solitary left posterior thigh mass measuring 1.5 cm in diameter, which was later shown by positron emission tomography-computed tomography (PET-CT) to have multiple simultaneous mediastinal lymph node metastases. Eleven months ago, the patient had undergone curative breast-conserving surgery (BCS) for cancer of her left breast. At that time, her tumor showed a triple negative phenotype. Initial PET-CT right before the BCS had shown no metastasis. After histological and radiologic evaluation for the metastases, she decided to have systemic chemotherapy.
Soft tissue is usually not the site of distant dissemination of solid tumors. It has been shown that intramuscular soft tissue metastatic nodules are painful, whereas soft tissue sarcomas scarcely produce pain, but grow slowly.(1) Therefore, painless soft tissue nodules, especially remote from the index tumor are usually regarded as being unrelated to the primary carcinoma. Commonly, some solid tumors, such as lung, kidney, and colon carcinomas, have been known to be a prevalent source of soft tissue metastasis.(2) To date, there have been no reports on metastatic breast cancer presenting as a painless soft tissue nodule residing subcutaneously at the posterior thigh, and found to have multiple mediastinal lymph node metastases verified by positron emission tomography-computed tomography (PET-CT).
As a result of an intense database search and to our understanding, this is the first report of its kind in Korea. We think it is very crucial to differentiate soft tissue metastasis from the index tumor and de novo soft tissue sarcoma given that the prognosis of each case may vary profoundly, especially after misdiagnosis. The onset of metastases into mediastinal lymph nodes and farther down to the posterior thigh seemed to be fairly rapid in this case, stressing the importance of a thorough follow-up in younger breast cancer patients who harbor a triple-negative phenotype.
Eleven months ago, a 31-yr-old woman visited our clinic presenting with a palpable breast lump in the left upper outer quadrant of her breast. After pathologic confirmation by core-needle biopsy, she decided to have breast-conserving surgery for her breast carcinoma. The pathological report indicated a triple-negative invasive ductal breast cancer without axillary lymph node metastasis. Her tumor measured 1.5 cm in maximum diameter and had no lymphatic, vascular, or perineural invasion. Immunohistochemical staining for estrogen receptor, progesterone receptor, HER2 receptor, Ki-67, and p53 mutation were negative for estrogen, progesterone, epidermal growth factor receptor (EGFR) and HER2 receptor, but not Ki-67>45%, and positive for p53 mutation and E-cadherin. All histologic and nuclear grades for her index tumor were grade 3.
After a thorough systemic evaluation including postoperative PET-CT (Figure 1A), she showed no evidence of distant tumor burden of any kind or anywhere at that time. After considerate discussion regarding systemic treatment for triple-negative breast cancer, she refused chemotherapy. On discharge after her curative operation, she was informed that she had T1N0M0 invasive ductal breast carcinoma. Six months after her curative operation, she showed no objective signs or subjective symptoms suggesting locoregional or systemic recurrence on follow-up studies including breast magnetic resonance imaging (MRI) encompassing the whole thoracic cavity and plain chest film.
Recently, she found a painless soft tissue mass which had been growing at her left posterior thigh for a month.
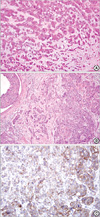
She visited our clinic ahead of her predetermined one yr follow-up. On physical examination, the mass measured about 1.2 cm in maximum diameter, but produced no pain while growing. Otherwise, she complained of nothing else. In order to form a correct histological diagnosis, an excisional biopsy was done under local anesthesia, soon after having a radiologic interpretation of PET-CT in order to rule out possible distant metastasis (Figure 1B). The soft tissue mass resided subcutaneously, right beneath the fat layer, but above the lower muscular layer (Figure 2A). Subsequent to verifying metastatic breast cancer through a permanent section due to the fact that it shared histologic feature and characteristics with the index tumor (Figure 2B), we tried to locate any other possible metastatic tumor burden in her body. Except for the left posterior thigh mass which was removed along with an ample safety margin, as well as several mediastinal lymph nodes located by PET-CT, nothing more could be found or suspected to harbor metastatic burden stemming from the previous breast cancer. Immunohistochemical stains for the metastatic thigh mass yielded negative for estrogen, progesterone, and HER2 receptor (Immunohistochemical staining ++, fluorescence in situ hybridization result was negative), but not for Ki-67>70%, and positive for p53 mutation, E-cadherin, and EGFR (Figure 2C). Systemic chemotherapy with docetaxel (75 mg/m2) and doxorubicin (50 mg/m2) was initiated after receiving the patient's informed consent. A thorough follow-up and continuing systemic treatment was recommended.
Breast cancer is one of the most dreadful cancers for women worldwide. In Korea, the incidence of breast cancer is reportedly rising.(3) Even though many new chemotherapeutic agents and treatment modalities have led to improved survival of breast carcinoma patients, we often unexpectedly have to face distant dissemination of this ailment. That is the reason why we have to thoroughly examine and follow-up patients utilizing a battery of tests, including radiologic work-up.
Breast cancer is known to metastasize to anywhere in the body, either by hematogenous or lymphatogenous routes. In this case, the predetermined first six-month follow-up studies (breast MRI and plain chest film) after curative operation produced no objective evidence of locoregional or distant metastasis, especially in the thorax. However, the patient noticed a small palpable but painless subcutaneous lobular nodule in her left posterior thigh which she recognized at ten months postoperative. Considering possible isolated soft tissue metastases directly from otherwise unremarkable cases of breast cancer is rare. However, in this case, the lobular characteristic of the soft tissue mass made us suspect the mass was metastatic or a different kind of primary malignancy mandating histological verification and systemic PET-CT scan. According to the PET-CT scan, the patient seemed to have numerous mediastinal lymph nodes metastases and possible pleural nodules suggesting metastases in addition to the isolated left posterior thigh uptake, indicating metastatic potential from the previously resected breast adenocarcinoma. Except for the uptake in the mediastinum and left posterior thigh, nothing else caused any suspicion of metastasis.
At this time, there have been only two large reports on distant soft tissue metastasis.(4,5) However, not a single report described subcutaneous nodules leading to the diagnosis of metastatic breast cancer. Most cases indicated skin, kidney, lung, colon, bone, ovary, or cervical cancer as primary tumors, with the clear exception of breast cancer. In this case, we presume mediastinal and possible pleural metastases progressed down to left posterior thigh, but we are unable to suggest the exact route to the spot without leaving tumor cells behind along the intervening normal-looking structures. Usually, more than half of the body mass reported resides in soft tissue and blood vessels may not be the route for this type of dissemination.(5) Therefore, usually the origin of the soft tissue mass is from the nearby surroundings. In addition, there may have been so many local or tissue factors identified that make it difficult for cancer cells to travel across the soft tissue.(6) The thigh has been known as one of the most commonly affected areas among soft tissues.(5) Further, physicians succeeded in locating the primary tumor sites about one-fifth of these kind of metastases; however, one-seventh of cases failed to be found with the origin.(5)
The thigh is not a usual site for the distant dissemination from the breast cancer; however, both painful and painless growing soft tissue masses, irrespective of their distance from the primary breast cancer, should be examined and biopsied to rule out possible metastatic breast cancer. The reason for this is that the prognosis of neglected soft tissue metastases from breast cancer has been death in a span of about five months.(4)
To our understanding, this is the first case of thigh metastasis from breast cancer. This presumably occurred after mediastinal lymph node metastasis; however, simultaneous metastasis into both the mediastinal lymphatic basin and left posterior thigh were theoretically possible through lymphatic channels. However, it is impossible to precisely determine the route of distant dissemination. Further, there are no sine qua non features that can confirm this nodule as metastatic breast cancer, except for the fact that it shared histologic features with the index breast cancer and no other primary cancer. Accordingly, we may suggest histological verification and systemic evaluation, whenever soft tissue masses are located elsewhere even after a curative breast cancer operation. In doing so, patients will be saved from dismal prognoses due to improper and delayed diagnosis and treatment.
Figures and Tables
Figure 1
(A) Positron emission tomography-computed tomography (PET-CT) scan taken right after a curative breast cancer operation. Postoperative PET-CT showed no distant dissemination from the primary left breast cancer. (B) PET-CT scan at follow-up. PET-CT scan taken eleven months after the curative operation showed several mediastinal lymph nodes and possible pleural nodules along with a left thigh mass, which were suggestive of distant metastasis from the primary breast cancer.

Figure 2
(A) Metastatic nodule at the left posterior thigh. Undifferentiated carcinoma cells are seen blending with the matrix material. The lacunar structure of cartilage is not present. (H&E stain, ×200). (B) Index tumor. Grade III invasive ductal carcinoma from the left upper breast with ductal carcinoma in situ (comedo type) (H&E stain, ×100). (C) Immunohistochemical staining (IHC) of human epidermal growth factor receptor in the metastatic nodule. The result of IHC was ++, which was later proven negative by fluorescence in situ hybridization (×200).
References
1. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop Relat Res. 1993. 296:213–217.

2. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997. 168:555–557.

3. The Korean Breast Cancer Society. Clinical characteristics of Korean breast cancer patients in 1998. J Korean Med Sci. 2000. 15:569–579.
4. Damron TA, Heiner J. Distant soft tissue metastases: a series of 30 new patients and 91 cases from the literature. Ann Surg Oncol. 2000. 7:526–534.

5. Plaza JA, Perez-Montiel D, Mayerson J, Morrison C, Suster S. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer. 2008. 112:193–203.
6. Herring CL Jr, Harrelson JM, Scully SP. Metastatic carcinoma to skeletal muscle: a report of 15 patients. Clin Orthop Relat Res. 1998. 272–281.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download