Abstract
Purpose
The aim of the study was to determine steroid sulfatase (STS) expression in endometrial cancer patients and its correlation with disease prognosis.
Materials and Methods
We conducted a retrospective study in 59 patients who underwent surgery with histologically confirmed endometrial cancer from January 2000 to December 2011 at Hanyang University Hospital. Immuno-histochemical staining of STS was performed using rabbit polyclonal anti-STS antibody.
Results
Sixteen of the 59 patients (27.1%) were positive for STS expression. Disease free survival (DFS) was 129.83±8.67 [95% confidence interval (CI): 112.84–146.82] months in the STS positive group (group A) and 111.06±7.17 (95% CI: 97.01–125.10) months in the STS negative group (group B) (p=0.92). Overall survival (OS) was 129.01±9.38 (95% CI: 110.63–147.38) months and 111.16±7.10 (95% CI: 97.24–125.07) months for the groups A and B, respectively (p=0.45). Univariate analysis revealed that FIGO stage and adjuvant therapy are significantly associated with DFS and OS. However, in multivariate analysis, FIGO stage and adjuvant therapy did not show any statistical significance with DFS and OS. STS was also not significantly associated with DFS and OS in univariate and multivariate analysis.
Estrogen and progesterone production is essential for normal proliferation of the endometrium. Previous studies have shown that continuous and excessive exposure to estrogen increases the risk of endometrial cancer.1234 However, there is a paradoxical increase of estrogen-dependent endometrial cancer in postmenopausal women who have stopped producing estrogen in the ovaries. Hence, intrinsic synthesis and metabolism of estrogen plays an important role in the prevalence and progression of endometrial cancer.5 Inhibition of sex hormones in patients with endometrial cancer is thus an important therapeutic goal to reduce levels of biologically significant estrogen and achieve better prognosis.67
Regulation of estrogen synthesis and action in the treatment of hormone-dependent breast cancer has been successful in the past.8 Tamoxifen, the estrogen receptor (ER) blocker has been standard treatment until now, and aromatase inhibitors (AIs) have been used as a first line therapy in metastatic and hormone-dependent breast cancer in postmenopausal women. AIs are also widely used currently, as first line therapy in early ER positive breast cancer in postmenopausal women. Unfortunately, despite advances in therapeutic strategies, the majority of breast cancer patients who undergo these treatments have progressive disease after a few years of a complete response.
Androgen receptor (AR) is expressed in >80% of ER positive postmenopausal breast cancer women. However, AR is also expressed in ER negative patients. Androstenediol (Adiol) can bind to ER, despite being an androgen, and results in an increase in the number of ER positive breast cancer cells. Although Adiol has a weak affinity to ER, high concentrations of Adiol of >100 fold can have the same effect as estradiol (E2).9 Clinical treatment with AIs inhibits the synthesis of E2 by >99%, but simultaneously sensitizes to very E2 low concentrations. Adiol-induced low E2 concentration potentially affects the progression of breast cancer.8 The androgen-induced activation of estrogen through AR in ER-negative breast cancer patients is via steroid sulfatase (STS). Therefore, inhibition of STS can reduce estrogen synthesis by Adiol.
Most STS inhibitor clinical studies in breast cancer patients are in the clinical trial phase, and an ongoing study on hormone-dependent endometrial cancer that is similar to breast cancer is in the animal study phase. To the best of our knowledge, there is no study on STS with human endometrial cancer tissue. Therefore, we investigated STS expression in human endometrial cancer tissue and determined its correlation with prognosis in patients with or without expression of STS.
We conducted a retrospective study in 59 patients who underwent surgery for endometrial cancer from January 2000 to December 2011 at Hanyang University Hospital. STS expression was confirmed through immuno-histochemical staining of sections from paraffin-embedded endometrial cancer tissue. We excluded patients who were diagnosed as recurrent endometrial cancer, transferred from another hospital after hysterectomy, and were lost to follow up after surgery. We evaluated patient characteristics, including age, parity, types of surgery, serum levels of CA125 and 19-9 before and after surgery, the International Federation of Gynecology and Obstetrics (FIGO) stage, histologic type and grade, and adjuvant therapies, such as chemotherapy, radiation therapy, and concurrent chemoradiation therapy (CCRT). In survival analysis, last day of treatment and follow up, day of recurrence, and day of death were investigated. The study was approved by the Institutional Review Board (Study approval No.: HYUH 2012-R-03).
Patients were followed up by physical examination and for tumor markers (CA125/19-9) every 3 months for 2 years, and every 6 months for the next 3 years. Chest X-ray, abdomino-pelvic computed tomography, and 18F-fluoro-D-glucose positron emission tomography-computed tomography (18FDG PET-CT) were performed annually. Recurrence of disease was defined as presence of tumor histologically or radiologically. Any suspicious lesion on CT scan was followed-up with CT scans every 3 months until recurrence was confirmed clinically. In cases of recurrence, 18FDG PET-CT was performed to locate other sites of recurrence, and all sites of recurrence were documented after 2006.
We performed total hysterectomy with bilateral salpingo-oophorectomy without lymphadenectomy in cases where the tumor mass was confined to the endometrium and there was no significant lymphadenopathy. Para-aortic lymphadenectomy was performed in cases where the tumor mass was ≥2 cm in size, or there were more than 1/2 invasions of the myometrium or positive pelvic lymphadenopathy in frozen sections.
We did not perform adjuvant treatment in patients with stage IA and grade 1, 2, or vaginal brachytherapy in patients with stage IA and grade 3, or stage IB. Patients with stage II received external pelvic radiotherapy and chemotherapy, and extended radiotherapy was given in patients with stage III or stage IV.
The most morphologically representative and non-necrotic area was carefully selected and marked on the hematoxylin-eosin stained slide. Two tissue cores (2 mm in diameter) were sampled from each paraffin block and assembled into a recipient paraffin block using a tissue microarray instrument (AccuMax array, ISU Abix, Seoul, Korea).
The 4-µm-thick tissue sections were cut from the tissue microarray blocks and deparaffinized with xylene and rehydrated with graded alcohol. Antigen retrieval was performed by microwaving the samples for 12 min in preheated 10 mM sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked with peroxidase blocking solution (DAKO, Carpinteria, CA, USA) for 10 min. The rabbit polyclonal anti-STS antibody (ab62219, Abcam, Cambridge, MA, USA) was diluted 1:100 and incubated at 4℃ for 16 h. The sections were then incubated with a peroxidase labeled anti-mouse/rabbit secondary antibody for 30 min (DAKO, Carpinteria, CA, USA). The samples were developed with DAB substrate (DAKO, Carpinteria, CA, USA) for 2 min and counterstained with Mayer's hematoxylin. Subsequently, standard procedure was used to dehydrate the slides and seal with coverslips. Negative controls were performed by omitting STS antibody during the primary antibody incubation. Normal placental tissue served as positive control.
Immunohistochemical staining of STS was evaluated semiquantitatively based on staining intensity and percentage of positive cells. The intensity score was based on the staining intensity as negative (0 point), weak (1 points), intermediate (2 points), and strong (3 points). A final score was then calculated by a labeling index with percentage of positive cells.10 Expression of STS was considered 'positive' when >5% of tumor cells had any cytoplasmic staining.1011
Group wise comparisons of categorical clinical characteristics were by the chi-square and Fisher's exact tests. For continuous variables, mean values were compared between the groups using the Mann-Whitney U-test. Disease-free survival (DFS) and overall survival (OS) were estimated using the Kaplan-Meier method, and hazard ratio estimates and confidence intervals (CI) were generated using the Cox proportional hazard model. Statistical significance was defined as 2-sided p value of <0.05. Data were analyzed using SPSS software for Windows (version 18.0; SPSS Inc., Chicago, IL, USA).
Fifty-nine patients were included in this study. Of these, 16 patients (27.1%) were STS positive (group A) (Fig. 1A) and 43 patients were STS negative (group B) (Fig. 1B). There was no significant difference in general characteristics between group A and group B. None of the patients received CCRT as an adjuvant treatment (Table 1). In the STS positive group, 8 patients were diagnosed as stage IA, and 3 patients were diagnosed as more than stage IB who were received adjuvant treatment; three patients were grade 1, 5 patients were grade 2 and 8 patients were grade 3. In the STS negative group, 26 patients were stage IA, and 6 patients were stage IB and IC who were received adjuvant treatment; eleven patients were grade 1 and 15 patients were grade 2 and 6 patients were grade 3.
Two of the 16 patients in group A had recurrence and 1 patient died. Six patients had recurrence and subsequent mortality in group B. Using Kaplan-Meier survival analysis, DFS (mean±standard deviation) was 129.83±8.67 (95% CI: 112.84–146.82) months in group A, and 111.06±7.17 (95% CI: 97.01–125.10) months in group B (p=0.92) (Fig. 2). OS (mean±standard deviation) was 129.01±9.38 (95% CI: 110.63–147.38) months in group A, and 111.16±7.10 (95% CI: 97.24–125.07) months in group B (p=0.45) (Fig. 3).
In univariate Cox proportional hazard analysis, FIGO stage (p=0.007 in DFS, p=0.006 in OS) and adjuvant treatment (p=0.034 in DFS, p=0.034 in OS) were significantly associated with DFS and OS. In multivariate analysis, however, FIGO stage and adjuvant treatment were not significantly associated with DFS and OS. The expression of STS was not statistically significant with DFS and OS (Table 2 and 3).
STS is a single enzyme that hydrolyses not only estrone sulfate (E1S) and estradiol sulfate (E2S), but also various steroid sulfates, such as dehydroepiandrosterone sulfate (DHEA-S) and cholesterol sulfate. Synthesis of sex hormones involves the conversion of androstenedione or testosterone to E2 or estrone (E1) by aromatase, and these hormones are converted by 17β-hydroxysteroid dehydrogenase (17βHSD). The inhibition of aromatase or 17βHSD at this point would inhibit the synthesis of E2 or E1. However, Adiol, which is synthesized from DHEA or DHEA-S via STS, is not inhibited and is eventually converted into estrogen.
The activity of STS is 12 times higher in endometrial cancer tissue, as compared to normal endometrial tissue,12 and is expressed up to 86% on immunohistochemical staining.13 Previous studies showed that the STS pathway is an important source of estrogen and STS inhibitors are effective in breast cancer. These results have led to the clinical use of STS and STS inhibitors in patients with another hormone-dependent cancer (i.e., endometrial cancer).
STX 64 is the only STS inhibitor that completed the phase I clinical trial among the developed STS inhibitors. In various in vivo tumor models, STX 64 showed a great inhibitory effect of estrogen activity.14 STX 213 is a second generation STS inhibitor that showed an eight times stronger effect than STX 64 in vitro to completely block estrogen activity. The most significant characteristic of second generation STS inhibitor is the long duration of STS inhibition. In the study with a mouse model, the time to recover of STS activity was 10 days in STX 213, as compared to 3 days in STX 64.1516 However, the clinical application of STS inhibitors is limited. DFS was reported from 2.8 months to 7 months in phase I of clinical trial with 14 breast cancer patients using STX 64.17
Although limited, clinical studies were conducted in breast cancer patients, but only animal studies were conducted in endometrial cancer patients. When STX 64 and STX 213 were administered orally to ovariectomized rats with endometrial cancer xenograft, the cancer cell growth was inhibited by 48% and 67%, respectively. Furthermore, cancer cell growth was inhibited up to 59% and serum estradiol was significantly reduced by STX 213 at 10 mg/kg daily.18
Unlike previous in vitro and in vivo studies, we used human endometrial cancer tissues. In this study, STS was expressed in sixteen patients out of 59 patients (27%), which was lower than the previous animal study with immuno-histochemical staining (86%). A possible explanation for this discrepancy might be the use of paraffin-embedded tissue post-formalin fixing vs. fresh endometrial cancer tissue. The use of rabbit polyclonal anti-STS antibody for STS detection might be another cause. Although previous study showed that the immune response was lower in the endometrium, as compared to human breast or hepatic tissue,19 there is no antibody with better response to STS detection.
Our results showed that the expression of STS did not affect DFS and OS in endometrial cancer patients. Although the effect was not statistically significant, survival was better in the STS positive group, unlike in the previous study conducted in breast cancer patients. In addition, FIGO stage and adjuvant treatment, which are known prognostic factors, were not associated with DFS and OS. The main reasons for this were the small sample size and the few events of recurrence and death. Other reasons were subtle differences between the two groups in the proportions of the cell types and in the numbers of patients receiving radiation therapy. Specifically, the STS negative group contained a larger number of patients with papillary serous adenocarcinoma and undergoing radiation therapy, and a previous study has shown that papillary serous adenocarcinoma has a poorer prognosis than endometroid adenocarcinoma.20 Also, radiation therapy has been considered the standard of adjuvant treatment, but the treatment failure rate in advanced stage patients is reportedly up to 67%.21
Another consideration is the sex hormone inhibitor with different response in breast and endometrium tissue. Tamoxifen has an anti-estrogen effect on breast tissue, but has an estrogen effect on endometrium tissue, which can cause endometrial hyperplasia or dysfunctional uterine bleeding and endometrial cancer.222324 On the other hand, raloxifene, another selective ER active substance, has a treatment effect on breast cancer and neutral effect on endometrial tissue.25 AIs also showed excellent clinical results in breast cancer, but there is only a minimal inhibitory effect on endometrial cancer.262728 These results can be explained by ER. The subtype of ER is divided into alpha receptor (ERα) and beta receptor (ERβ), both of which have a different distribution in each organ.29 ERα is dominant in breast tissue, while ERβ is dominant in endometrial tissue.30 Therefore, the effect of the same sex hormone inhibitor can differ in breast and endometrial cancer. Another study showed that the mutation of exon 5, 8, and 36 in ERα may lead to lower anti-cancer effects in endometrial cancer.313233
Theoretically, the clinical effect of STS inhibitor in endometrial cancer is expected to be significant. The effect would be greater if there is concurrent inhibition of aromatase and 17βHSD in the main pathway of estrogen synthesis and STS. However, we did not show the effect of the expression of STS in endometrial cancer as a prognostic factor. Although these findings were rather disappointing, they warrant future endometrial cancer trials with STS including ER, more specific antibody and endometrial cell biological markers.
In conclusion, STS was detected in approximately 27% human endometrial cancer tissue by immuno-histochemical staining. However, STS expression was not significantly associated with DFS and OS. Therefore, STS as a prognostic factor in patients with endometrial cancer is questionable, hence requires more study and a careful result-based approach.
Figures and Tables
Fig. 1
The results of immunohistochemical staining (×400) of steroid sulfatase. (A) Positive. (B) Negative.

Fig. 2
Kaplan-Meier estimates of disease free survival between STS positive and STS negative in patients with endometrial cancer. STS, steroid sulfatase.

Fig. 3
Kaplan-Meier estimates of overall survival between STS positive and STS negative in patients with endometrial cancer. STS, steroid sulfatase.

Table 1
General Characteristics in Patients with Endometrial Cancer
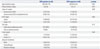
Table 2
Association of Clinicopathologic Variables with Disease-Free Survival in Patients with Endometrial Cancer

Table 3
Association of Clinicopathologic Variables with Overall Survival in Patients with Endometrial Cancer

Notes
References
1. Sherman ME, Sturgeon S, Brinton L, Kurman RJ. Endometrial cancer chemoprevention: implications of diverse pathways of carcinogenesis. J Cell Biochem Suppl. 1995; 23:160–164.

2. Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000; 13:295–308.

4. Kelsey JL, LiVolsi VA, Holford TR, Fischer DB, Mostow ED, Schwartz PE, et al. A case-control study of cancer of the endometrium. Am J Epidemiol. 1982; 116:333–342.

5. Ryan KJ. Editorial: Cancer risk and estrogen use in the menopause. N Engl J Med. 1975; 293:1199–1200.
6. Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev. 2005; 26:171–202.

7. Woo LW, Purohit A, Potter BV. Development of steroid sulfatase inhibitors. Mol Cell Endocrinol. 2011; 340:175–185.

8. Geisler J, Sasano H, Chen S, Purohit A. Steroid sulfatase inhibitors: promising new tools for breast cancer therapy? J Steroid Biochem Mol Biol. 2011; 125:39–45.

9. Masamura S, Santner SJ, Heitjan DF, Santen RJ. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab. 1995; 80:2918–2925.

10. Sasano H, Frost AR, Saitoh R, Harada N, Poutanen M, Vihko R, et al. Aromatase and 17 beta-hydroxysteroid dehydrogenase type 1 in human breast carcinoma. J Clin Endocrinol Metab. 1996; 81:4042–4046.

11. Utsunomiya H, Suzuki T, Kaneko C, Takeyama J, Nakamura J, Kimura K, et al. The analyses of 17beta-hydroxysteroid dehydrogenase isozymes in human endometrial hyperplasia and carcinoma. J Clin Endocrinol Metab. 2001; 86:3436–3443.

12. Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, et al. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987; 47:5616–5619.
13. Utsunomiya H, Ito K, Suzuki T, Kitamura T, Kaneko C, Nakata T, et al. Steroid sulfatase and estrogen sulfotransferase in human endometrial carcinoma. Clin Cancer Res. 2004; 10:5850–5856.

14. Purohit A, Woo LW, Potter BV, Reed MJ. In vivo inhibition of estrone sulfatase activity and growth of nitrosomethylurea-induced mammary tumors by 667 COUMATE. Cancer Res. 2000; 60:3394–3396.
15. Foster PA, Newman SP, Chander SK, Stengel C, Jhalli R, Woo LL, et al. In vivo efficacy of STX213, a second-generation steroid sulfatase inhibitor, for hormone-dependent breast cancer therapy. Clin Cancer Res. 2006; 12:5543–5549.

16. Foster PA, Chander SK, Parsons MF, Newman SP, Woo LW, Potter BV, et al. Efficacy of three potent steroid sulfatase inhibitors: pre-clinical investigations for their use in the treatment of hormone-dependent breast cancer. Breast Cancer Res Treat. 2008; 111:129–138.

17. Stanway SJ, Purohit A, Woo LW, Sufi S, Vigushin D, Ward R, et al. Phase I study of STX 64 (667 Coumate) in breast cancer patients: the first study of a steroid sulfatase inhibitor. Clin Cancer Res. 2006; 12:1585–1592.

18. Foster PA, Woo LW, Potter BV, Reed MJ, Purohit A. The use of steroid sulfatase inhibitors as a novel therapeutic strategy against hormone-dependent endometrial cancer. Endocrinology. 2008; 149:4035–4042.

19. Selcer KW, Difrancesca HM, Chandra AB, Li PK. Immunohistochemical analysis of steroid sulfatase in human tissues. J Steroid Biochem Mol Biol. 2007; 105:115–123.

20. Greggi S, Mangili G, Scaffa C, Scala F, Losito S, Iodice F, et al. Uterine papillary serous, clear cell, and poorly differentiated endometrioid carcinomas: a comparative study. Int J Gynecol Cancer. 2011; 21:661–667.

21. Ayeni TA, Bakkum-Gamez JN, Mariani A, McGree ME, Weaver AL, Haddock MG, et al. Comparative outcomes assessment of uterine grade 3 endometrioid, serous, and clear cell carcinomas. Gynecol Oncol. 2013; 129:478–485.

22. Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004; 94:256–266.

23. Neven P, Vergote I. Controversies regarding tamoxifen and uterine carcinoma. Curr Opin Obstet Gynecol. 1998; 10:9–14.

24. Slomovitz BM, Sun CC, Ramirez PT, Bodurka DC, Diaz P, Lu KH. Does tamoxifen use affect prognosis in breast cancer patients who develop endometrial cancer? Obstet Gynecol. 2004; 104:255–260.

25. DeMichele A, Troxel AB, Berlin JA, Weber AL, Bunin GR, Turzo E, et al. Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol. 2008; 26:4151–4159.

26. Berstein L, Maximov S, Gershfeld E, Meshkova I, Gamajunova V, Tsyrlina E, et al. Neoadjuvant therapy of endometrial cancer with the aromatase inhibitor letrozole: endocrine and clinical effects. Eur J Obstet Gynecol Reprod Biol. 2002; 105:161–165.

27. Barker LC, Brand IR, Crawford SM. Sustained effect of the aromatase inhibitors anastrozole and letrozole on endometrial thickness in patients with endometrial hyperplasia and endometrial carcinoma. Curr Med Res Opin. 2009; 25:1105–1109.

28. Ma BB, Oza A, Eisenhauer E, Stanimir G, Carey M, Chapman W, et al. The activity of letrozole in patients with advanced or recurrent endometrial cancer and correlation with biological markers--a study of the National Cancer Institute of Canada Clinical Trials Group. Int J Gynecol Cancer. 2004; 14:650–658.

29. Couse JF, Curtis Hewitt S, Korach KS. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J Steroid Biochem Mol Biol. 2000; 74:287–296.

30. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997; 138:863–870.

31. Bryant W, Snowhite AE, Rice LW, Shupnik MA. The estrogen receptor (ER)alpha variant Delta5 exhibits dominant positive activity on ER-regulated promoters in endometrial carcinoma cells. Endocrinology. 2005; 146:751–759.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download