Abstract
Purpose
To evaluate the influence of preoperative mechanical bowel preparation (MBP) based on the occurrence of anastomosis leakage, surgical site infection (SSI), and severity of surgical complication when performing elective colorectal surgery.
Materials and Methods
MBP and non-MBP patients were matched using propensity score. The outcomes were evaluated according to tumor location such as right- (n=84) and left-sided colon (n=50) and rectum (n=100). In the non-MBP group, patients with right-sided colon cancer did not receive any preparation, and patients with both left-sided colon and rectal cancers were given one rectal enema before surgery.
Results
In the right-sided colon surgery, there was no anastomosis leakage. SSI occurred in 2 (4.8%) and 4 patients (9.5%) in the non-MBP and MBP groups, respectively. In the left-sided colon cancer surgery, there was one anastomosis leakage (4.0%) in each group. SSI occurred in none in the rectal enema group and in 2 patients (8.0%) in the MBP group. In the rectal cancer surgery, there were 5 anastomosis leakages (10.0%) in the rectal enema group and 2 (4.0%) in the MBP group. SSI occurred in 3 patients (6.0%) in each groups. Severe surgical complications (Grade III, IV, or V) based on Dindo-Clavien classification, occurred in 7 patients (14.0%) in the rectal enema group and 1 patient (2.0%) in the MBP group (p=0.03).
Preoperative mechanical bowel preparation (MBP) has a few theoretical advantages.1,2,3,4 First, MBP removes fecal bacteria, which reduces the risk of complications from infections. Second, removing the feces makes it easier to manipulate the bowel and lowers the risk of unwanted fecal spillage into the abdominal cavity. Third, feces inside the large intestine may cause anastomotic disruption; hence, MBP aims to reduce the risk of feces related complications.
However, in 1972, Hughes5 questioned the efficacy of MBP when performing a colectomy. Additionally, the potential benefits of MBP have not been continuously reproduced.6,7 Even more, some studies have suggested that the MBP approach should be abandoned due to its harmful effects of MBP in terms of higher anastomosis leakage rate8,9,10 or higher wound infection rate.11 However, Slim, et al.12 did not find that there was a negative impact of MBP on anastomotic leakage in MBP versus non-MBP patients (p=0.46), but instead that surgical site infections were more common in MBP patients than in non-MBP patients (p=0.02).
In regard to rectal surgery, a non-MBP strategy has not been well studied. According to a Cochrane review, there were no differences in anastomotic leakage and wound infection rate between MBP and non-MBP patients after low anterior resection.13 However, a recent trial showed higher overall infectious morbidity in rectal cancer surgery without MBP.14 Due to these contradictions, the majority of colorectal surgeons still perform MBP prior to colorectal surgery
The purpose of this study was to evaluate the impact of preoperative MBP based on the incidences of anastomosis leakage, surgical site infection (SSI), and the severity of surgical complication based on Dindo-Clavien classification15 when performing elective colorectal surgery.
From September 1, 2010 to August 31, 2012, a total of 380 patients were enrolled in the study and underwent elective colorectal surgery for colorectal cancer at a tertiary referral center. According to the use of MBP, the data of 234 patients from this patient population was selected using propensity score matching. This study was approved by the Institutional Review Board (YWMR-12-5-043).
MBP had been performed routinely in the colorectal cancer clinic until 2009. In 2010, MBP became a selective procedure, and because of this, patients that were enrolled in the study chose whether or not they wanted to receive an MBP after a thorough explanation of the MBP procedure. MBP was not performed on patients that had difficulty ingesting 4 liters (L) of polyethylene glycol (PEG) solution. Additionally, in cases where the surgery was planned within one week after the initial diagnostic colonoscopy, MBP was not used in patients that did not want to take the PEG solution repeatedly.
The following criteria were used to include patients in the study: histopathologically confirmed adenocarcinoma, elective surgery, and a complete colonoscopy examination of the entire colon. The criteria used for exclusion from the study included an emergency surgery, recurrent colorectal cancer, synchronous primary colorectal cancer, no colonoscopy passage into the proximal portion of the lesion, clinically early lesions less than 2 cm in size that required intraoperative colonoscopy, and no primary anastomosis.
The right-sided colon was defined as the cecum, ascending colon, hepatic flexure, and transverse colon. The left-sided colon was defined as the splenic flexure, descending colon, and sigmoid colon.
This was a retrospective analysis of prospectively collected data. Because of the inability to randomly allocate patients to either receive MBP or to not receive MBP before surgery, a propensity score was used to control for selection bias. In observational studies, there are often significant differences between characteristics of a treatment group and control group. These differences must be adjusted in order to reduce treatment selection bias and determine treatment effect. Propensity scores are used in observational studies to reduce selection bias by matching different groups based on these propensity score probabilities, rather than matching patients on the values of the individual covariates. A propensity score is simply a probability that a subject would be assigned to a specific group, and matching subjects on propensity scores produces comparison groups of subjects who would be equally likely to have been assigned to the study's group or condition.16
First, univariate analysis was performed to compare patient characteristics between MBP and non-MBP patients. Next, the propensity score (the predicted probability of receiving MBP, P (Y=1│X=x) for each of the 380 patients was estimated using a logistic regression model. Then, covariates were matched based on the predicted probability to reduce selection bias. Age, gender, American Society of Anesthesiologists, pathological Tumor-Node-Metastasis stage, body mass index, laparoscopy, and duration of prophylactic antibiotics have been used as a matching variable for right and left colon cancer. The use of preoperative chemoradiation was added as matching variables in addition to aforementioned matching variables for rectal cancer (Table 1).
The primary endpoint was to evaluate anastomotic leakage rate and the secondary endpoints were to evaluate SSI rate and severity of surgical complications based on Dindo-Clavien classification according to the use of MBP
Severity of postoperative complications was assessed based on Dindo-Clavien classification grading system which was designed for grading surgical complication by severity (Grade I, II, III, IV, and V).15 Anastomotic leakage was defined as clinical signs of gas, pus, or fecal drainage based on physical examination or disruption of anastomosis based on imaging studies such as computed tomography or a distal cologram. Based on Dindo-Clavien classification, a grade II leakage was defined as a condition requiring pharmacological treatment. A grade III leakage was defined as a condition requiring surgical, endoscopic, or radiological intervention. According to the Centers for Disease Control and Prevention guidelines, SSI was categorized as superficial incisional SSI, deep incisional SSI, and organ (or space) SSI.17 All intra- and postoperative complications were prospectively recorded for 30 days following the surgery.
Patients with MBP fasted one day before surgery and ingested 4 L of PEG solution. A rectal glycerine enema was performed twice, once in the afternoon and once in the evening on the day before the surgery. In the non-MBP group, patients with the right-side colon cancers did not receive any oral solution and fasted from midnight the night before the surgery. Patients with left-side colon and rectal cancers received one glycerine enema in the evening prior to the surgery and fasted from midnight to the night before surgery. First-generation cephalosporin (cefazolin) was used as a prophylactic antibiotic and was administered just before the start of surgery. Antibiotic treatment was maintained for 24 to 48 hours after surgery.
All surgeries were performed by a colorectal specialist. Complete mesocolic excision and central vascular ligation were performed in the patients with colon cancer and, in patients with rectal cancer, a high ligation of the inferior mesenteric artery and total mesorectal excision were performed. The surgeon performed stapled colorectal anastomosis for low anterior resection and hand-sewn coloanal anastomosis for ultra-low anterior resection. A defunctioning ileostomy was created based on the surgeon's discretion.
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20.0. (IBM, Armonk, NY, USA). For the analysis, both Student's t-test for continuous variables and a chi-square test (Fisher's exact test) for categorical variables were performed. A p-value of less than 0.05 was considered to be statistically significant.
There were no differences of anastomosis leakage, SSI, and Grade III, IV, or V complications based on Dindo-Clavien classification.
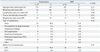
Detailed complications based on Dindo-Clavien classification were recorded in the following manner: in the non-MBP group, 1 patient developed pneumonia, whereas another patient developed small bowel obstruction and subsequently underwent adhesiolysis. In the MBP group, 2 patients developed deep incisional SSI which required surgical repair, 1 patient developed organ (or space) SSI which required surgical drainage, and 2 patients developed small bowel obstructions which required adhesiolysis (Table 2).
There were no differences of anastomosis leakage, SSI, and Grade III, IV, or V complications.
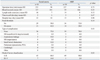
Detailed complications based on Dindo-Clavien classification were recorded in the following manner: in the rectal enema group, one patient developed a cerebral hemorrhage, and another patient developed an anastomosis leakage and subsequently underwent anastomosis revision and transverse loop colostomy. In the MBP group, one patient died after developing a pulmonary thromboembolism (Table 3).
There were five incidences of anastomosis leakage (10.0%) in the rectal enema group and two incidences (4.0%) in the MBP group (p=0.24). There was no difference of SSI between two groups. Grade III, IV, or V complications based on Dindo-Clavien classification occurred in 7 patients (14.0%) from the rectal enema group and 1 patient (2.0%) from the MBP group (p=0.03).
Detailed complications based on Dindo-Clavien classification were recorded in the following manner: in the rectal enema group, 3 patients developed small bowel obstruction, and 1 of these patients underwent a Hartmann procedure while the two remaining patients underwent adhesiolysis. Additionally, 3 patients in the rectal enema group developed anastomosis leakage for which 1 patient that had undergone an initial loop ileostomy underwent laparotomy and transanal repair, and the 2 other patients underwent laparotomy and loop ileostomy. One patient developed deep incisional SSI which required surgical repair. In the MBP group, 1 patient developed anastomosis leakage which required a Hartmann procedure (Table 4).
The major finding of this study was that right-sided and left-sided colon cancer surgery could be performed safely without an MBP or by using an enema-only approach. However, rectal cancer surgery after an enema-only could be dangerous because of higher risks of severe postoperative complications (Grade III, IV, and V) based on Dindo-Clavien classification.
This study has some strengths. First, this study was designed to differentiate the type of anastomosis such as ileo-colic, colo-colic, and colo-rectal anastomosis, and the outcomes were evaluated according to tumor location such as right- and left-sided colon and rectum. Second, MBP and non-MBP patients were matched according to propensity score to reduce selection bias.
In this study, non-MBP patients that underwent ileocolic anastomosis for right-sided colon cancer did not receive any preoperative bowel preparation, but the non-MBP patients that underwent colo-colic (or colo-rectal anastomosis) for left-sided colon and rectal cancers received a rectal enema to remove solid feces. This procedure was performed because intraluminal solid feces in the proximal and distal colon may prevent manipulation of the circular stapler and may be entrapped with the staple line during firing.8,18
The primary endpoint of this study was to evaluate anastomotic leakage rate according to the use of MBP. The results showed that there were no difference of anastomosis leakage among non-MBP, rectal enema, and MBP groups when performing right- and left-sided colonic surgery. These results are supported by the evidence that primary colonic repair is safe in selected patients with traumatic colonic injury,19,20 and primary colo-colic anastomosis has been safely performed in patients with obstructing colon cancer.21,22
In rectal surgery, our results indicated that, the incidence of anastomosis leakage was higher in the non-MBP group (10%) compared to the MBP group (4%), even though this result was not statistically significant. Contant, et al.23 found that the rate of anastomotic leakage did not differ according to the use of MBP. However, MBP patients had a lower rate of intra-abdominal abscesses after anastomotic leakage than non-MBP patients. Similarly, in the present study, the rectal enema group contained 3 patients that experienced anastomosis leakage, one of whom had an initial loop ileostomy, which revealed symptomatic leakage and caused the three patients to eventually undergo a laparotomy. This result can be explained by the fact that pre-existing solid feces in the proximal colon may increase the symptoms of leakage. Therefore, routine fecal diversion may be of help in non-MBP patients that undergo rectal surgery.24
Van't Sant, et al.25 reported that there were no significant differences with respect to anastomosis leakage or septic complications between non-MBP and MBP patient groups. In contrast, in 2010, Bretagnol, et al.14 reported that there was a higher rate of 30-day overall morbidity and infectious complications in non-MBP compared to MBP patients after rectal cancer surgery. Moreover, although not statistically significant, their study also indicated that there was a higher rate of anastomotic leakage (19% vs. 10%) and peritonitis (7% vs. 2%) in non-MBP patients. Similarly, our study indicated that, in rectal surgery, severe complications that are defined as greater than grade III occurred more often in the rectal enema group.
The relationship between the use of an MBP and anastomosis failure is not clearly understood. When an ileocolic anastomosis is performed, liquid ileal contents pass through the anastomosis. The use of MBP does not greatly affect the small bowel contents and thus the relationship between the use of MBP and anastomotic failure seems to be minimal in ileo-colic anastomosis. In cases of the colo-colic or colo-rectal anastomosis without an MBP, proximal colonic feces pass through the anastomosis. The direct effect of feces on the anastomosis has not been clearly defined. Some insight has been gained based on an experimental animal study, where the negative impact of feces on rat colonic healing was studied.4 In the rat analysis, anastomotic failure occurred more commonly in a feces-loaded colon after a left-sided colectomy. O'Dwyer, et al.26 investigated the influence of MBP on anastomosis after low anterior resection in dogs, where it was concluded that anastomotic bursting pressures were significantly higher in the MBP group. In addition, pelvic abscess and death from peritonitis were lower in the MBP groups (6%) compared to the unprepared group (29%).
The secondary endpoints of this study were to evaluate SSI rate. In the present study, the results indicated that there was no statistically significant difference in the occurrence of SSI between the non-MBP and the MBP groups. Intraoperative spillage of bowel contents following MBP has been suggested as a risk factor for infectious complication. Mahajna, et al.27 suggested that liquid bowel contents after an MBP increase the chance of intraoperative spillage, and that the spillage may lead to postoperative infectious complications. Zmora, et al.28 found that intestinal spillage in the MBP group occurred more frequently, but the rates of surgical infections did not differ between the two groups. In our opinion, intestinal stapling to remove a colorectal specimen has been more widely used in recent years and surgeons are familiar with stapled ileo-colic anastomosis and double stapling technique in low anterior resection. Accordingly, the exposure time of intestinal contents to operative field is relatively short and the risk of spillage during elective surgery seems to be low. Moreover, poor colonic preparation after an MBP has been suggested as a risk factor for anastomosis leakage in elective anterior resection.29 However, based on our experiences, if colonoscopy or gastroscopy pass through the colonic lumen which contains the tumor, the incidence of unacceptably poor colonic preparation, such as a severely distended or unprepared colon, seems to be uncommon. Even if patients have a gas or fluid-filled colon, the intestinal content could be easily decompressed through a newly made small hole in the proximal colon stump without causing major spillage.
This study has limitations. The present study included single-center series and was retrospective in nature. However, the patients were allocated based on propensity score matching, and the outcome data were collected prospectively.
In conclusion, right- and left-sided colon cancer surgery can be performed safely without an MBP with respect to anastomosis leakage, SSI and the severity of surgical complication. In the rectal cancer surgery, a rectal enema only could be dangerous due to the higher incidences of grade III, IV, or V complication based on Dindo-Clavien classification.
Figures and Tables
Table 2
Intra- and Postoperative Outcomes of Right-Sided Colon Cancer Surgery Based on the Use of Mechanical Bowel Preparation (MBP)

ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A100054). IY Kim and SK Ahn designed this study. EH Choi, HJ Kwon, and SK Ahn collected the data. IY Kim, EH Choi, and YW Kim drafted the manuscript. YW Kim and HJ Kwon participated in critical revision of the manuscript.
References
1. Nichols RL, Condon RE. Preoperative preparation of the colon. Surg Gynecol Obstet. 1971; 132:323–337.
2. Wolters U, Keller HW, Sorgatz S, Raab A, Pichlmaier H. Prospective randomized study of preoperative bowel cleansing for patients undergoing colorectal surgery. Br J Surg. 1994; 81:598–600.

3. Clarke JS, Condon RE, Bartlett JG, Gorbach SL, Nichols RL, Ochi S. Preoperative oral antibiotics reduce septic complications of colon operations: results of prospective, randomized, double-blind clinical study. Ann Surg. 1977; 186:251–259.

4. Smith SR, Connolly JC, Gilmore OJ. The effect of faecal loading on colonic anastomotic healing. Br J Surg. 1983; 70:49–50.

5. Hughes ES. Asepsis in large-bowel surgery. Ann R Coll Surg Engl. 1972; 51:347–356.
6. Irving AD, Scrimgeour D. Mechanical bowel preparation for colonic resection and anastomosis. Br J Surg. 1987; 74:580–581.

7. The 75th meeting of the Surgical Research Society. 9-10 January 1992, England. Abstracts. Br J Surg. 1992; 79:441–468.
8. Bucher P, Mermillod B, Gervaz P, Morel P. Mechanical bowel preparation for elective colorectal surgery: a meta-analysis. Arch Surg. 2004; 139:1359–1364.

9. Slim K, Vicaut E, Panis Y, Chipponi J. Meta-analysis of randomized clinical trials of colorectal surgery with or without mechanical bowel preparation. Br J Surg. 2004; 91:1125–1130.

10. Guenaga KF, Matos D, Castro AA, Atallah AN, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2003; CD001544.

11. Platell C, Hall J. What is the role of mechanical bowel preparation in patients undergoing colorectal surgery? Dis Colon Rectum. 1998; 41:875–882.

12. Slim K, Vicaut E, Launay-Savary MV, Contant C, Chipponi J. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg. 2009; 249:203–209.

13. Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011; CD001544.

14. Bretagnol F, Panis Y, Rullier E, Rouanet P, Berdah S, Dousset B, et al. Rectal cancer surgery with or without bowel preparation: The French GRECCAR III multicenter single-blinded randomized trial. Ann Surg. 2010; 252:863–868.
15. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
16. Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999; 150:327–333.

17. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992; 13:606–608.

18. Bucher P, Gervaz P, Soravia C, Mermillod B, Erne M, Morel P. Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery. Br J Surg. 2005; 92:409–414.

19. Curran TJ, Borzotta AP. Complications of primary repair of colon injury: literature review of 2,964 cases. Am J Surg. 1999; 177:42–47.

20. Conrad JK, Ferry KM, Foreman ML, Gogel BM, Fisher TL, Livingston SA. Changing management trends in penetrating colon trauma. Dis Colon Rectum. 2000; 43:466–471.

21. Mealy K, Salman A, Arthur G. Definitive one-stage emergency large bowel surgery. Br J Surg. 1988; 75:1216–1219.

22. White CM, Macfie J. Immediate colectomy and primary anastomosis for acute obstruction due to carcinoma of the left colon and rectum. Dis Colon Rectum. 1985; 28:155–157.

23. Contant CM, Hop WC, van't Sant HP, Oostvogel HJ, Smeets HJ, Stassen LP, et al. Mechanical bowel preparation for elective colorectal surgery: a multicentre randomised trial. Lancet. 2007; 370:2112–2117.

24. Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007; 246:207–214.

25. Van't Sant HP, Weidema WF, Hop WC, Oostvogel HJ, Contant CM. The influence of mechanical bowel preparation in elective lower colorectal surgery. Ann Surg. 2010; 251:59–63.
26. O'Dwyer PJ, Conway W, McDermott EW, O'Higgins NJ. Effect of mechanical bowel preparation on anastomotic integrity following low anterior resection in dogs. Br J Surg. 1989; 76:756–758.
27. Mahajna A, Krausz M, Rosin D, Shabtai M, Hershko D, Ayalon A, et al. Bowel preparation is associated with spillage of bowel contents in colorectal surgery. Dis Colon Rectum. 2005; 48:1626–1631.

28. Zmora O, Mahajna A, Bar-Zakai B, Rosin D, Hershko D, Shabtai M, et al. Colon and rectal surgery without mechanical bowel preparation: a randomized prospective trial. Ann Surg. 2003; 237:363–367.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download