Abstract
Purpose
Approximately 50% to 70% of women with polycystic ovary syndrome (PCOS) have some degree of insulin resistance, and obesity is known to worsen insulin resistance. Many metabolic consequences of PCOS are similar to those of obesity; therefore, defining the cause of insulin resistance in women can be difficult. Our objective was to clarify the factors contributing to insulin resistance in PCOS.
Materials and Methods
We consecutively recruited 144 women with PCOS [age: 26±5 yr, body mass index, body mass index (BMI): 24.4±4.0 kg/m2] and 145 controls (age: 25±5 yr, BMI: 23.0±3.6 kg/m2), and divided them into overweight/obese (ow/ob, BMI ≥23 kg/m2) and lean (BMI <23 kg/m2) groups. Anthropometric measures and a 75-g oral glucose tolerance test were performed, and insulin sensitivity index (ISI) was calculated as an index of insulin sensitivity. Factors predictive of ISI were determined using regression analysis.
Results
ISI was significantly lower in both lean and ow/ob women with PCOS compared to BMI-matched controls (p<0.05). Increasing BMI by 1 kg/m2 decreased ISI by 0.169 in PCOS patients (p<0.05) and by 0.238 in controls (p<0.05); there was no significant difference between these groups. In lean PCOS patients and lean controls, BMI had no effect on ISI. Multiple regression analysis revealed that PCOS status (β=-0.423, p<0.001) and BMI (β=-0.375, p<0.001) were significantly associated with ISI.
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders, affecting 5-17% of women of reproductive age, and it is characterized by hyperandrogenism and ovulatory dysfunction.1-4 Insulin resistance is one of the potential underlying causes of PCOS, and is present in at least 50% of women with PCOS.5 Insulin resistance could also cause or exacerbate the clinical manifestations of PCOS, including ovulation dysfunction, hirsutism and metabolic disturbances.2,6
Controversy exists as to whether lean women with PCOS are insulin resistant.6-13 It is uncertain whether insulin resistance in PCOS is an intrinsic defect of the disease or is secondary to obesity. Obesity has long been recognized as one of the features of PCOS, and 40-80% of women with PCOS are overweight or obese.14,15 The mechanisms by which obesity influences the pathophysiology and clinical manifestations of PCOS are not completely understood, but obesity has an important impact on the severity of hyperandrogenism, menstrual irregularities and insulin resistance.16 Even modest weight loss has been shown to result in significant improvements in insulin resistance in women with PCOS.17,18 However, many metabolic consequences of PCOS are similar to those of obesity; therefore, defining the cause of the insulin resistance has proven difficult. In addition, it is unclear whether the exacerbation of insulin resistance, owing to a rise in adiposity, differs between obese and lean women.
In the present study, we evaluated changes in insulin sensitivity as a function of body mass index (BMI), as well as factors associated with insulin sensitivity, to determine whether insulin resistance is an intrinsic defect of PCOS or occurs secondary to obesity.
We recruited 144 women with PCOS from endocrinology and gynecology clinics at Ewha Womans University Mokdong Hospital from March 2003 through March 2007. In accordance with the National Institute of Child Health and Human Disease criteria,19 the diagnosis of PCOS was based on the following: 1) hyperandrogenism (total testosterone ≥67 ng/dL or free testosterone20 ≥0.84 ng/dL, above the 95th percentile of 1120 regular cycling healthy women) and 2) ovulatory dysfunction (less than 8 menstrual cycles per year). Individuals with specific disorders, such as adult-onset congenital adrenal hyperplasia, hyperprolactinemia, and androgen-secreting neoplasia, were excluded. One hundred and forty five regular cycling women were recruited as the control group by advertisement, and none of them had a family history of diabetes or PCOS. All women treated with drugs known to influence glucose tolerance (e.g., steroids, oral contraceptives, metformin or thiazide diuretics) before starting the study were excluded. On the basis of their BMI, women with PCOS and healthy controls were divided into two groups: lean (with a BMI <23 kg/m2) and overweight/obese (ow/ob) (with a BMI ≥23 kg/m2). The criteria for obesity were based on Asia-Pacific criteria.21 The institutional review board of the Ewha Womans University Mokdong Hospital approved the study protocol, and written informed consent was obtained from all of the participants.
Weight and height were measured for all subjects, and BMI was calculated as weight (kg)/height (m).2 Waist circumference was also measured on bare skin at the narrowest indentation between the 10th rib and the iliac crest, at mid-respiration. Blood pressure was determined as the mean of two manual sphygmomanometer readings with the patient in the sitting position. After overnight fasting for at least 8 h, a venous blood sample was taken from all subjects on the third day of their follicular phase of menstrual cycle. In the case of women with amenorrhea, blood was sampled considering the ovarian morphology investigated by ultrasound. Ultrasound examination was performed with a 7-MHz transvaginal transducer (Logic 400 General Electric, Milwaukee, WI, USA) or transrectally for virginal women.
Total testosterone levels were measured by the chemiluminescent immunoassay method using a commercially available kit (Siemens, Tarrytown, NY, USA), and sex hormone-binding globulin (SHBG) levels were measured by immunoradiometric assay using a commercial kit (Diagnostic Products Corporation, Los Angeles, CA, USA). Free testosterone levels were calculated using the formula available on the web site of the International Society for Study of the Aging Male (http://www.issam.ch/freetestos.htm) for total testosterone, SHBG and albumin levels in the same sample from each subject.20
The 75-g oral glucose tolerance test (OGTT) was performed in the morning after overnight fasting. A polyethylene catheter was placed into the antecubital vein before the test. After 30 minutes of supine rest, venous blood samples were drawn at baseline and 120 minutes after the 75-g glucose load. Plasma glucose levels were measured by the glucose oxidase method (Beckman Model Glucose Analyzer 2, Fullerton, CA, USA) and insulin levels were measured by radioimmunoassay using commercially available kit (Biosource, Nivelles, Belgium). Insulin sensitivity was estimated by ISIest-OGTT (ISI) according to the following formula: ISI=0.157-4.576×10-5×I120-0.00519×G90-0.000299×I0 (I120; post-load insulin at 120 minutes, G90; post-load glucose at 90 minutes, and I0; fasting insulin).22 Although the hyperinsulinemic-euglycemic clamp technique is the gold standard for measuring insulin sensitivity, it is difficult to perform; therefore, we used ISI, which showed a significant correlation with the M value obtained from the euglycemic clamp,22 as a marker of insulin sensitivity.
Statistical evaluation was performed with the SPSS 18.0 software package for Windows (IBM corporation, Chicago, IL, USA). Quantitative variables are given as means±standard deviation. The Kolmogorov-Smirnov statistic was used to test continuous variables for normality, and logarithmic transformation was applied as needed to ensure the normal distribution of skewed variables.
Comparisons of two groups with different parameters were made by Analysis of Covariance for adjusting age. To examine the effects of PCOS, BMI and their interaction on parameters, two-way Analysis of Variance (ANOVA) was conducted. To examine the influence of an increase in BMI on insulin sensitivity, linear regression analysis was performed. Multiple regression analysis was performed to establish which variables predicted insulin resistance independently. Two-tailed p-values of <0.05 were considered to be significant.
One hundred and forty-four women with PCOS were divided into two groups according to their BMI. Sixty-nine of these women were lean and 75 were ow/ob. The mean age of the women with PCOS was 26±5 years, and the mean BMI was 24.0±4.0 kg/m2. Of the 145 controls (mean age 25±5 years, mean BMI 23.0±3.6 kg/m2), 84 were lean and 61 were ow/ob.
The clinical and metabolic characteristics of the women with PCOS and the controls are shown in Table 1. The age did not differ between these two groups, but the BMI of women with PCOS was higher than that of controls (p=0.02). Women with PCOS had a higher systolic blood pressure (SBP, p<0.001), diastolic blood pressure (DBP, p<0.001), total testosterone level (p<0.001), and free testosterone level (p<0.001) than healthy controls, but their SHBG levels (p<0.001) were lower. The BMI did not differ between the two lean groups (PCOS and healthy controls) and between the two ow/ob groups (PCOS and healthy control). In both lean and ow/ob women with PCOS, the SBP (p<0.05), DBP (p<0.05), total testosterone levels (p<0.001), and free testosterone levels (p<0.001) were higher than those in their healthy counterparts, and SHBG levels (p<0.001) were lower (Table 1).
In women with PCOS, 2-h post-load glucose (p<0.001) and 2-h post-load insulin (p<0.05) were higher than those in controls. In lean women with PCOS, 2-h post-load glucose (p<0.001) and 2-h post-load insulin (p<0.001) were higher than those in lean controls. In ow/ob women with PCOS, 2-h post-load glucose (p<0.001), fasting insulin (p<0.05), and 2-h post-load insulin (p<0.05) were higher than those in controls. Insulin sensitivity as determined by ISI was significantly lower in women with PCOS than in controls, and both lean and ow/ob women with PCOS also showed lower ISI values than women in the corresponding BMI-matched control groups (p<0.001). Two-way ANOVA revealed that PCOS had significant effects on SBP, DBP, total testosterone, free testosterone, SHBG, 2-h post-load glucose, 2-h post-load insulin, and ISI. BMI had significant effects on SBP, DBP, free testosterone, SHBG, fasting glucose, 2-h post-load glucose, fasting insulin, 2-h post-load insulin, and ISI. The interaction between PCOS and BMI had significant effects on fasting insulin and 2-h post-load insulin.
An increase of 1 kg/m2 in BMI reduced ISI by 0.169 among all PCOS patients (p<0.05) and by 0.238 among all controls (p<0.05), and there was no significant difference between these two groups. When the ow/ob and lean groups were analyzed, BMI had no significant effect on ISI in lean women with PCOS or in the lean controls. In ow/ob women, an increase of 1 kg/m2 in BMI reduced the ISI by 0.146 among PCOS patients (p<0.05) and by 0.278 among controls (p<0.05), and there was no significant difference between these two groups (Table 2).
We applied multiple linear regression analysis to identify the main determinants of insulin sensitivity. BMI and PCOS status were significantly associated with ISI (p<0.05), and PCOS status was the most powerful determining factor (Table 3).
The objectives of this study were to evaluate the influence of increased adiposity on insulin sensitivity, and to identify the factors associated with insulin sensitivity. In this study, we found that insulin sensitivity was significantly lower in both lean and ow/ob women with PCOS than in BMI-matched controls, and insulin resistance was shown to be an intrinsic defect in women with PCOS.
The etiology of PCOS is complex and multifactorial, and insulin resistance could be one of the underlying causes.23,24 Overall, approximately 50-70% of women with PCOS can be considered to have insulin resistance,25,26 and insulin resistance is exacerbated in obese women with PCOS.8,27-29 There is some controversy about whether lean women with PCOS are insulin resistant.8,10,12,13,18,30,31 In Asia, the prevalence of obesity in women with PCOS is lower than in western women with PCOS.14,15 Given the lower prevalence of obesity in Asian women with PCOS than in those of other ethnicities,32 an understanding of whether lean Asian women with PCOS are significantly more insulin resistant than BMI-matched female counterparts and whether insulin resistance could be exacerbated by weight gain is important. In our study, even lean women with PCOS showed significantly lower insulin sensitivity than BMI-matched controls, and this result corresponded with an earlier study that reported that PCOS was associated with decreased insulin sensitivity in both obese and non-obese patients.8,13 This suggests that insulin resistance is an intrinsic characteristic of the disease. A hyperinsulinemic-euglycemic clamp is generally regarded as a reference method for assessing insulin sensitivity; however, this method is laborious and expensive. In contrast, OGTT, the most commonly used method for evaluating whole body glucose tolerance, is simple and cheap, and many formulas for insulin sensitivity index obtained from OGTT have been developed. We show to use ISI, which showed a significant correlation with the M value from the euglycemic clamp, as a marker of insulin sensitivity.22
We used linear regression analysis to evaluate the influence of increased BMI on insulin sensitivity. The results showed that BMI is a significant determinant of insulin sensitivity, suggesting that adiposity is an important factor in the pathogenesis of insulin resistance in both women with PCOS and in controls. To determine whether the association between BMI and insulin sensitivity differed according to adiposity, we analyzed the subjects in two groups: an ow/ob and a lean group. In ow/ob women with PCOS and ow/ob controls, BMI was a significant determinant of insulin sensitivity, but this was not the case in lean women with PCOS and in lean controls. Therefore, it appeared that the significant association between the BMI on insulin sensitivity was only present in ow/ob women. Although it did not reach statistical significance, the absolute value of the increase in ISI as a function of BMI increase was higher in controls than in women with PCOS. This result supports the hypothesis that insulin resistance is an intrinsic abnormality in this disease.
In the general populations, obesity and insulin resistance increase the risk of type 2 diabetes and cardiovascular disease. Likewise, in individuals with PCOS, obesity worsens insulin resistance and exacerbates metabolic abnormalities.33,34 Furthermore, women with PCOS have an increased risk of developing type 2 diabetes and cardiovascular disease. Dyslipidemia is common in PCOS patients compared with weight-matched controls,35,36 and women with PCOS also develop abnormal glucose metabolism at a younger age and may demonstrate a more rapid conversion from impaired glucose tolerance to type 2 diabetes than healthy women.37 The results of the present study of young women with PCOS corresponded to the results of earlier studies that reported that women with PCOS have a greater cardiovascular disease risk.38,39 In our study, women with PCOS had a higher SBP, DBP, 2-h post-load glucose, insulin, and triglyceride level than BMI-matched controls.
Although insulin resistance is a common feature of PCOS, not all women with PCOS are insulin resistant. Obesity appears to exert an important effect on the manifestations of PCOS, and the degree of obesity is positively associated with an increase in insulin resistance. However, many metabolic consequences of PCOS are similar to those of obesity, making it difficult to elucidate the cause of insulin resistance. In our study, both BMI and PCOS status were significantly associated with insulin sensitivity, suggesting that obesity and PCOS have a deleterious additive effect on insulin sensitivity.
The clinical features of PCOS are heterogeneous and may change throughout an individual's life, from adolescence to post menopause.16 This change is largely dependent on the influence of weight gain and metabolic alterations. Thus, weight gain is an important contributor to PCOS phenotype. To prevent metabolic dysfunction, it is therefore important to prevent weight gain in women with PCOS, especially in those who are already overweight.
In conclusion, our results demonstrate that Asian women with PCOS have intrinsic insulin resistance, and adiposity exacerbates such insulin resistance, especially in ow/ob women. Therefore, long-term lifestyle modification may be necessary to prevent weight gain, especially in ow/ob Asian women with PCOS.
Figures and Tables
Table 1
Clinical and Metabolic Characteristics in Women with PCOS and Controls According to Adiposity
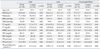
BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; FPI, fasting plasma insulin; FT, free testosterone; ISI, insulin sensitivity index; PCOS, polycystic ovary syndrome; PPG, post-load 2-hr plasma glucose; PPI, post-load 2-hr plasma insulin; SBP, systolic blood pressure; SHBG, sex hormone binding globulin; TT, total testosterone.
Data are mean±SD. Age-adjusted.
References
1. Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999. 84:4006–4011.

2. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998. 83:3078–3082.

3. Boyle JA, Cunningham J, O'Dea K, Dunbar T, Norman RJ. Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin, Australia. Med J Aust. 2012. 196:62–66.

4. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010. 25:544–551.

5. Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril. 2002. 77:1095–1105.

6. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006. 91:4237–4245.

7. Dunaif A. Insulin action in the polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999. 28:341–359.

8. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989. 38:1165–1174.

9. Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010. 95:2038–2049.

10. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997. 18:774–800.

11. Venkatesan AM, Dunaif A, Corbould A. Insulin resistance in polycystic ovary syndrome: progress and paradoxes. Recent Prog Horm Res. 2001. 56:295–308.

12. Morin-Papunen LC, Vauhkonen I, Koivunen RM, Ruokonen A, Tapanainen JS. Insulin sensitivity, insulin secretion, and metabolic and hormonal parameters in healthy women and women with polycystic ovarian syndrome. Hum Reprod. 2000. 15:1266–1274.

13. Toprak S, Yönem A, Cakir B, Güler S, Azal O, Ozata M, et al. Insulin resistance in nonobese patients with polycystic ovary syndrome. Horm Res. 2001. 55:65–70.

14. Franks S. Polycystic ovary syndrome: a changing perspective. Clin Endocrinol (Oxf). 1989. 31:87–120.

15. Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987. 65:499–507.

16. Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006. 113:1148–1159.

17. Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 1992. 36:105–111.

18. Holte J, Bergh T, Berne C, Wide L, Lithell H. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1995. 80:2586–2593.

19. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004. 81:19–25.
20. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999. 84:3666–3672.

21. Wen CP, David Cheng TY, Tsai SP, Chan HT, Hsu HL, Hsu CC, et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr. 2009. 12:497–506.

22. Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000. 23:295–301.

23. Escobar-Morreale HF, Luque-Ramírez M, San Millán JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev. 2005. 26:251–282.

25. Carmina E, Koyama T, Chang L, Stanczyk FZ, Lobo RA. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol. 1992. 167:1807–1812.

26. Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998. 83:2694–2698.

27. Marsden PJ, Murdoch A, Taylor R. Severe impairment of insulin action in adipocytes from amenorrheic subjects with polycystic ovary syndrome. Metabolism. 1994. 43:1536–1542.

28. Morales AJ, Laughlin GA, Bützow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996. 81:2854–2864.

29. Ciaraldi TP, el-Roeiy A, Madar Z, Reichart D, Olefsky JM, Yen SS. Cellular mechanisms of insulin resistance in polycystic ovarian syndrome. J Clin Endocrinol Metab. 1992. 75:577–583.

30. Holte J, Bergh T, Berne C, Berglund L, Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. J Clin Endocrinol Metab. 1994. 78:1052–1058.

31. Cibula D. Is insulin resistance an essential component of PCOS?: the influence of confounding factors. Hum Reprod. 2004. 19:757–759.

32. Park HR, Choi Y, Lee HJ, Oh JY, Hong YS, Sung YA. The metabolic syndrome in young Korean women with polycystic ovary syndrome. Diabetes Res Clin Pract. 2007. 77:Suppl 1. S243–S246.

33. Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995. 10:2107–2111.

34. Kiddy DS, Sharp PS, White DM, Scanlon MF, Mason HD, Bray CS, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clin Endocrinol (Oxf). 1990. 32:213–220.

35. Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. 2005. 90:5711–5716.

36. Talbott E, Clerici A, Berga SL, Kuller L, Guzick D, Detre K, et al. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol. 1998. 51:415–422.

37. Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999. 22:141–146.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download