Abstract
Objective
To investigate the usefulness of targeted ultrasound (US) in the identification of additional suspicious lesions found by magnetic resonance (MR) imaging in breast cancer patients and the changes in treatment based on the identification of the lesions by the use of targeted US.
Materials and Methods
One-hundred forty nine patients who underwent breast MR imaging for a preoperative evaluation of breast cancer between January 2002 and July 2004 were included in the study. We searched all cases for any additional lesions that were found initially by MR imaging and investigated the performance of targeted US in identifying the lesions. We also investigated their pathological outcomes and changes in treatment as a result of lesion identification.
Results
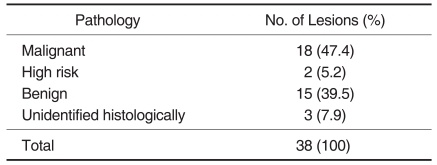
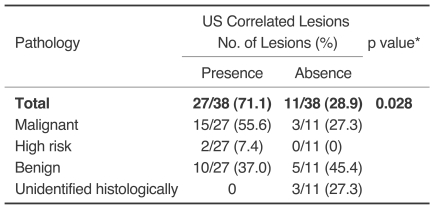
Of the 149 patients with breast cancer, additional suspicious lesions were detected with MR imaging in 62 patients (42%). Of the 69 additional lesions found in those 62 patients, 26 (38%) were confirmed as cancers by histology. Thirty-eight lesions in 31 patients were examined with targeted US and were histologically revealed as cancers in 18 (47%), high risk lesions in two (5%), benign lesions in 15 (39%), and unidentified lesions in three (8%). The cancer rate was statistically higher in lesions with a US correlate than in lesions without a US correlate (p = 0.028). Of 31 patients, the surgical plan was altered in 27 (87%). The use of targeted US justified a change in treatment for 22 patients (81%) and misled five patients (19%) into having an unnecessary surgical excision.
The high sensitivity of magnetic resonance imaging (MRI) to detect breast cancer enhances the evaluation of multifocal or multicentric lesions. Despite the occasional presence of additional suspicious lesions detected only by MRI, MR-guided percutaneous biopsies have not yet prevailed all over the world, because of a lack of commercially available equipment, a lack of real-time feedback, and the time-consuming procedure (1, 2). However, if careful reexamination with another conventional modality is used to visualize the additional MR detected lesions, this would allow a conventional means of biopsy guidance.
Often, MRI results in a change to surgical treatment plans based on the presence of additional suspicious lesions in patients with breast cancer. Considering the scheduling constrictions regarding surgical time, the fastest and most readily available method is needed for the preoperative localization of additional MR lesions, if possible, as well as histological confirmation. Ultrasound (US) dominates image guidance for breast interventions with its practical aspects and cost effectiveness. Nevertheless, few studies have reported on the usefulness of directed US investigations of breast lesions that were first detected with MRI in determining the probability of cancer and in the discovery of US correlates (3, 4). It is important to know the value and limitations of ultrasound in the evaluation of additional suspicious MR lesions in this special group. The purpose of our study was to investigate the usefulness of targeted US for identifying additional suspicious lesions found on MRI only for the preoperative evaluation of breast cancer and to determine whether the use of preoperative breast targeted US had an impact on subsequent surgical management.
The institutional review board approved this study and did not require the approval of patients nor their informed consent for review of their images and records. From January 2002 to July 2004, 303 women underwent breast MRI examinations were initially considered for this study. The primary inclusion criterion of this retrospective analysis was a preoperative MRI in patients with histologically confirmed breast cancer. Among the 303 women, 213 patients had confirmed breast cancer. Of these patients, 61 who underwent neoadjuvant chemotherapy were excluded because of a possible chemotherapeutic effect causing different kinetics and morphology of suspicious lesions (5). Three patients who did not undergo surgery in our institution were also excluded. The remaining 149 patients were included in this study.
The average age of the patients in the present study was 50 years (range 23 to 79 years). Indications for an MR examination included preoperative staging before planned breast conserving therapy to exclude multicentricity in patients who had one mammographic or sonographic suspicious lesion (n = 123), a search for an occult tumor with axillary metastases of suspected breast origin (n = 5) and a postoperative examination to rule out residual disease (n = 21).
A review of the MR images and final reports by an experienced radiologist specialized in breast imaging was conducted. We searched the cases with additional suspicious breast lesions that were initially detected by MRI. By the definition of Liberman et al. (6), MR lesions were considered to be additional sites if they were located in a different breast quadrant than the index cancer, if they were in the same quadrant but were separated from the index cancer by at least 1.0 cm of intervening normal-appearing tissue on MRI, or if they were in the same quadrant and contiguous with the index cancer but extended at least 4.0 cm beyond the site of the index cancer. Typically benign appearing enhancing lesions, i.e. those with a circumscribed margin or delayed enhancement were excluded.
If MRI revealed additional suspicious breast lesions other than the index cancer, a targeted US performed by the radiologist who interpreted the MR images was recommended to look for a US correlates amenable to further biopsy or localization. Although there were no standardized protocols to follow, a targeted US examination by the radiologists who had knowledge of the MRI findings was performed for the clinical and mammographical occult lesions. When targeted US was performed, the additional suspicious breast lesions were classified into two groups based on whether or not they had a US correlates. We investigated their pathological outcomes and changes in treatment for these patients. We compared the rate of identifying additional cancers between the lesions with and without a US correlate using the Fisher's exact test.
The 31 patients that did not receive targeted US consisted of two groups; one of the groups in whom the breast US was performed before the breast MR imaging and did not correlate with any additional detectable lesion by MRI and the other group with mammographic microcalcifications, which were not detected initially and did correlate with the MRI lesions. These lesions were confirmed by surgical excision or were followed with a subsequent examination.
Breast MRI was performed with the patient prone in a 1.5-T commercially available imager (Signa; GE Medical System, Milwaukee, WI) with the use of a dedicated surface breast coil. The MRI sequence used in this study included a fat suppressed axial fast spin echo T2-weighted sequence (4000/120, repetition time msec/echo time msec) and fat-suppressed unilateral sagittal dynamic imaging. Contralateral sagittal dynamic imaging was obtained on the next day. Dynamic imaging was performed with a T1-weighted three-dimensional, fat-suppressed fast spoiled gradient-echo sequence (17.3/1.3 ms; flip angle, 30°) three times (one before and two after a rapid bolus injection of 0.1 mmol/kg gadopentetate dimeglumine [Magnevist; Berlex, Wayne , NJ]) (7). Section thickness was held between 1.0-2.0 mm without an intersection gap with a 256 × 192 matrix, an 18-24 cm field of view, and a scan time of 3-4 minutes. Two sequential post-contrast scans were obtained without a break, beginning immediately after the saline flush. Standard subtraction images were obtained by subtracting the precontrast images from the early peak (or serial) postcontrast image on a pixel-by-pixel basis. Reverse subtraction images were obtained by subtracting the last postcontrast image from the early peak postcontrast image. If the lesion demonstrated a kinetic pattern showing an early rise and an early washout, the lesion would be observed with the remaining high signal intensity on reverse subtraction images. We regarded these lesions as kinetically suspicious lesions. If any kinetic curves were equivocal, the morphologic features were considered together. If the lesion has a spiculate or an irregular margin, we regarded it as a morphological suspicious lesion. If a lesion had morphologic features that indicated it was probably benign but reverse subtraction images demonstrated a high signal intensity lesion, this was also considered to be suspicious (8). Criteria for the features of suspicious non-mass-like enhancements included linear, ductal, and segmental patterns. Maximum intensity projection images were reformed from the subtraction images.
Breast US examinations were performed by one of four breast imaging radiologists using a 7-10 MHz linear transducer (Logiq 700; General Electric, Milwaukee, WI, or HDI 5000; Philips Medical Systems, Bothell, WA). At the time of breast US examination, prior MR images were made available for direct correlation.
Thirty-eight lesions in 31 patients were performed with targeted US. Of 21 patients with correlated suspicious enhancing lesions that were evident on US, eight underwent US-guided core biopsy for the suspicious lesion and 10 underwent US-guided localization, one with US-guided hook-wire localization and the remaining nine with US-guided carbon marking (9). In the residual three patients, two patients had a scheduled mastectomy due to the extensive extent of an index cancer and one had breast conserving surgery because of the lesion localized 1 cm just inferior to the index cancer within the same quadrant.
In the 10 patients without a US correlate, the lesions in six patients could not be localized. Of the four that underwent a US-guided localization, US-guided needle localization was used in one and carbon marking in three. Although lesions are not defined on US with certainty, localizations were performed in five lesions of four patients for subtle sonographic lesions likely consistent with the additional suspicious MR lesions. As the lesions without a US correlate could not be localized, careful histological examinations were requested at the area of any additional MR lesions.
Of the 149 total patients for whom MRI was used for preoperative evaluation of breast cancer, 69 additional suspicious breast lesions were detected by MRI in 62 patients (42%). Of those 69 lesions, 26 (38%) were confirmed as cancers by histology. By MRI, 48 of 69 lesions (70%) revealed a mass, and 21 (30%) demonstrated nonmass-like enhancements and the average diameter of the 69 additional enhancing lesions in these 62 patients was 1.1 cm (range, 0.4-5.0 cm).
In addition, of these 62 patients with 69 lesions, 57 (92%) had a single additional lesion, four (6%) had two lesions, and one (2%) had four lesions. Among 69 lesions, additional cancers were diagnosed in 26 (38%) in 23 patients. Contralateral enhancing lesions were detected in ten patients (16%), of which confirmed cancers in three patients (5%).
Of the 62 patients with additional lesions, 31 patients with 38 lesions underwent targeted US. Histological findings of these 38 lesions in 31 patients performed with targeted US revealed cancers in 18 (47%) lesions, high-risk lesions in two (5%), benign lesions in 15 (39%), and unidentified lesions in three (8%) (Table 1). The latter three lesions that were not confirmed by histological examination were removed by a mastectomy in one patient and breast conservative surgery with a wide extent in two patients.
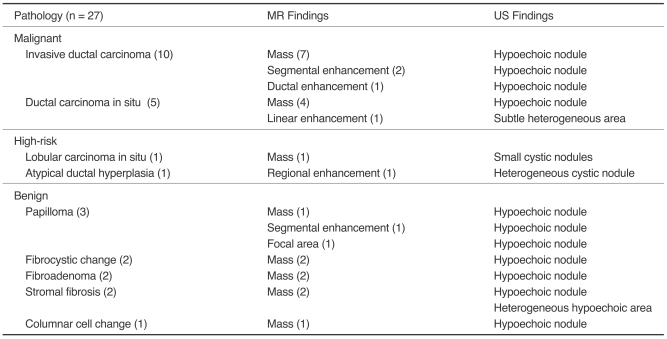
Of the 38 lesions examined with targeted US, 27 (71%) had a US correlate, which included 15 (56%, 15/27) cancers (Fig. 1), two (7%, 2/27) high-risk lesions (lobular carcinoma in situ and atypical ductal hyperplasia in each one) and ten (37%, 10/27) benign lesions (Fig. 2). Of the 11 lesions without a US correlate, three (27%, 3/11) proved to be cancerous (Fig. 3) and five (45%, 5/11) were benign as determined by mastectomy or breast conserving surgery with additional excision (Fig. 4), however, three (27%, 3/11) were not confirmed by histology. The cancer rate was statistically higher in lesions with a US correlate than in ones without a US correlate (p = 0.028) (Table 2). Of 15 cancers with a US correlate US, ten (67%) were confirmed as invasive ductal carcinoma, and five (33%) as ductal carcinoma in situ. Of the three cancers without a US correlate, two (67%) were ductal carcinoma in situ, and one (33%) as an invasive ductal carcinoma. The difference between invasive and in situ carcinoma was not statistically significant (p = 0.528). Of US correlate lesions identified by histology, the high-risk lesions included lobular carcinoma in situ and atypical ductal hyperplasia for each one. The benign lesions were diagnosed as papilloma in three, fibroadenoma, stromal fibrosis, fibrocystic disease in two each, and columnar cell change in one. Imaging findings and pathological results of US correlate lesions are summarized in Table 3.
Of 31 patients who underwent targeted US, the surgical plan was altered in 27 patients (87%) and not in four patients. Targeted US justified a change in treatment for 22 patients (81%) and misled five patients (19%) into an unnecessary surgical excision.
Of 21 patients with US correlates, the surgical treatment plan for 19 patients was altered preoperatively with the consent of the patient. These 19 patients included 14 with additional cancers, two with high-risk lesions and three with benign lesions (one fibroadenoma and two intraductal papillomas). Two patients with high-risk lesions underwent conserving surgery and a wider excision. Even though the US-guided core biopsy for three patients confirmed benign lesions, these patients underwent conserving surgery and a wider excision, because the surgeon did not want to defer the operation because of a personal concern of the patient or delayed pathological results with the consensus of the patient. Of the ten patients without US correlates, surgical treatment was changed in eight patients. However, three patients for whom cancers were confirmed subsequently underwent mastectomy at the discretion of surgeon (two), and conservative surgery with a wide excision during the operation (one). Five patients underwent unnecessary wide excision due to the difficulty in localizing the additional lesions.
In 13 (76%) of 17 patients (14 with US correlates and 3 without US correlates) in whom MRI detected additional cancers, the surgery was converted to mastectomy in ten or additional contralateral conserving surgery in three.
Breast MRI is generally accepted as the preferred diagnostic method for patients with breast cancer because of its high sensitivity (10-12). In contrast, the specificity of breast MR images remains highly variable (37-100%) (10, 11). In situations where there is a high probability of cancer such as preoperative staging, use of MRI can be very helpful. In the current study, the detection of additional lesions by MRI in breast cancer patients was frequent (42%). Among the 69 additional lesions detected by MRI, 26 (38%) were shown to be cancers following a histological examination.
Because of the need to biopsy and localize these lesions initially seen only on MRI, and until recently, the lack of biopsy systems that were MR compatible and commercially available, we designed an alternative method. If a lesion that is detected only on MRI can be found on US through careful re-examination, the visualization of the lesion on US will enable a US-guided intervention by a skilled radiologist and allow the patients to possibly avoid a multi-step surgery. Some investigators have previously reported on the reliability of targeted ultrasound for suspicious MRI-detected lesions (13-15). Because targeted US might be complementary to a subjective US by referencing MR imaging, this technical procedure is more helpful in reducing the number of missed cancers than using US alone or using US before MRI. In our study, targeted US found 71% of the additional lesions seen only on MR in breast cancer patients. In comparison to a previous report where 23% of the cases were detected, we suggest the basis for these differing results include the selection of patients that had a high probability of cancer, the more frequent use of bilateral whole breast ultrasound due to unfamiliarity with a skillful MR-guided biopsy technique, and an advantage in lesion detection due to the relatively smaller-sized breasts of Asian women included in the study that allowed routine use of a smaller film size (eg. 18 × 24 cm) introduced for mammograms (3). Biopsy or localization was successfully completed in all patients with a US correlate, and 15 (71%) of these patients had additional sites of cancer subsequently identified by MRI.
Of these 31 patients who underwent targeted US, carcinoma was found in 56% of patients that had a US correlate; in comparison, cancer was identified in 27% of patients lacking a US correlate. This difference was statistically significant (p = 0.028). Similar to a previous study, a US correlate was more often observed for cancers in the present study (3). Twenty lesions (74%) out of 27 with a US correlate were detected as a mass (or small nodule) on MRI and were observed more often for invasive forms (67%) than for in situ forms (33%) (p = 0.528).
Additional lesions that are verified by MRI may lead to either a justified surgical excision or over-treatment. In a previous study on preoperative MRI, planned surgical management was altered in 26% of the cases (16). In our study, we demonstrated that the targeted US results altered subsequent surgical management from what had been planned initially for 27 (87%) of all 31 patients who underwent targeted US. Targeted US in addition to MRI seems to contribute to an alteration in the surgical plan. Targeted US justified the change in treatment for 22 patients (81%) and misled five patients (19%) to unnecessary surgical excision. The five over-treated patients who underwent additional treatment had lesions without a US correlate.
A retrospective study such as this has deficiencies. First, histological confirmation was absent or unavailable in some instances, because of the inability to conduct MRI-guided percutaneous biopsy. Thus, excision of lesions without a US correlate sometimes resulted in sacrificing large amounts of normal tissue to examine an MR-suspicious area. This is a serious drawback for targeted US. Therefore, we speculate that an MR-guided biopsy provides the unique ability to identify these lesions that are not visualized by US (17, 18). Second, although US in this study was performed by four skilled breast imaging radiologists and carefully correlated with additional imaging studies, US interpretation is highly dependent upon both the operator and the equipment. As for the US findings in our study, radiologists tend to search for a sonographic hypoechoic lesion in order for it to be considered as suspicious. There may be a chance of missing the additional lesion that would be seen as a hyperechoic or isoechoic lesion by targeted US.
It has been our experience that if a suspicious lesion in patients with breast cancer is additionally depicted only on MRI, re-examination with another modality, specifically ultrasound, can improve the accuracy of identification and characterization of the lesion, thereby increasing confidence in the application of an intervention. However, our results also highlighted the need of MR-guided biopsies in some cases.
In conclusion, targeted US can play a useful role in the evaluation of additional suspicious MR lesions in breast cancer patients, but is limited in lesions without a US correlate.
References
1. van den Bosch MA, Daniel BL. MR-guided interventions of the breast. Magn Reson Imaging Clin N Am. 2005; 13:505–517. PMID: 16084416.

2. Atalar E, Menard C. MR-guided interventions for prostate cancer. Magn Reson Imaging Clin N Am. 2005; 13:491–504. PMID: 16084415.

3. LaTrenta LR, Menell JH, Morris EA, Abramson AF, Dershaw DD, Liberman L. Breast lesions detected with MR imaging: utility and histopathologic importance of identification with US. Radiology. 2003; 227:856–861. PMID: 12773685.

4. Sim LS, Hendriks JH, Bult P, Fook-Chong SM. US correlation for MRI-detected breast lesions in women with familial risk of breast cancer. Clin Radiol. 2005; 60:801–806. PMID: 15978891.

5. Londero V, Bazzocchi M, Del Frate C, Puglisi F, Di Loreto C, Francescutti G, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004; 14:1371–1379. PMID: 14986052.

6. Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR Am J Roentgenol. 2003; 180:901–910. PMID: 12646427.

7. Choi N, Han BK, Choe YH, Kim HS. Three-phase dynamic breast magnetic resonance imaging with two-way subtraction. J Comput Assist Tomogr. 2005; 29:834–841. PMID: 16272861.

8. Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999; 211:101–110. PMID: 10189459.

9. Mullen DJ, Eisen RN, Newman RD, Perrone PM, Wilsey JC. The use of carbon marking after stereotactic large-core-needle breast biopsy. Radiology. 2001; 218:255–260. PMID: 11152811.

10. Gilles R, Guinebretiere JM, Lucidarme O, Cluzel P, Janaud G, Finet JF, et al. Nonpalpable breast tumors: diagnosis with contrast-enhanced subtraction dynamic MR imaging. Radiology. 1994; 191:625–631. PMID: 8184038.

11. Harms SE, Flamig DP, Hesley KL, Meiches MD, Jensen RA, Evans WP, et al. MR imaging of the breast with rotating delivery of excitation off resonance: clinical experience with pathologic correlation. Radiology. 1993; 187:493–501. PMID: 8475297.

12. Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001; 220:13–30. PMID: 11425968.

13. Beran L, Liang W, Nims T, Paquelet J, Sickle-Santanello B. Correlation of targeted ultrasound with magnetic resonance imaging abnormalities of the breast. Am J Surg. 2005; 190:592–594. PMID: 16164928.

14. Obdeijn IM, Brouwers-Kuyper EM, Tilanus-Linthorst MM, Wiggers T, Oudkerk M. MR imaging-guided sonography followed by fine-needle aspiration cytology in occult carcinoma of the breast. AJR Am J Roentgenol. 2000; 174:1079–1084. PMID: 10749254.

15. Panizza P, De Gaspari A, Vanzulli A, Rodighiero M, Camalori M, Del Maschio A. Accuracy of post-MR imaging second-looksonography in previously undetected breast lesions. Radiology. 1997; 205(Suppl):489. (Abstract).
16. Bedrosian I, Mick R, Orel SG, Schnall M, Reynolds C, Spitz FR, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003; 98:468–473. PMID: 12879462.

17. Heywang-Kobrunner SH, Heinig A, Schaumloffel U, Viehweg P, Buchmann J, Lampe D, et al. MR-guided percutaneous excisional and incisional biopsy of breast lesions. Eur Radiol. 1999; 9:1656–1665. PMID: 10525886.
18. Kuhl CK, Morakkabati N, Leutner CC, Schmiedel A, Wardelmann E, Schild HH. MR imaging-guided large-core (14-gauge) needle biopsy of small lesions visible at breast MR imaging alone. Radiology. 2001; 220:31–39. PMID: 11425969.

Fig. 1
A 34-year-old woman with a 3-cm-sized palpable cancer of the right breast.
A, B. A dynamic enhanced and subtracted T1-weighted sagittal MR image shows a 3 cm, irregular-shaped, enhancing malignant mass (arrow) (A). A small subareolar enhancing nodule (arrowhead) is additionally noted in the subareolar region in a different plane (B).
C. A high signal intensity subareolar nodule (arrowhead) is noted on the reverse subtracted image.
D. Targeted US depicts a 0.7 cm sized hypoechoic nodule (arrowhead) between the nipple and a palpable malignant mass (arrow). A US-guided biopsy of an additional lesion revealed an infiltrating ductal carcinoma. The surgical plan was changed from conservative breast surgery to a modified radical mastectomy with the consent of the patient.
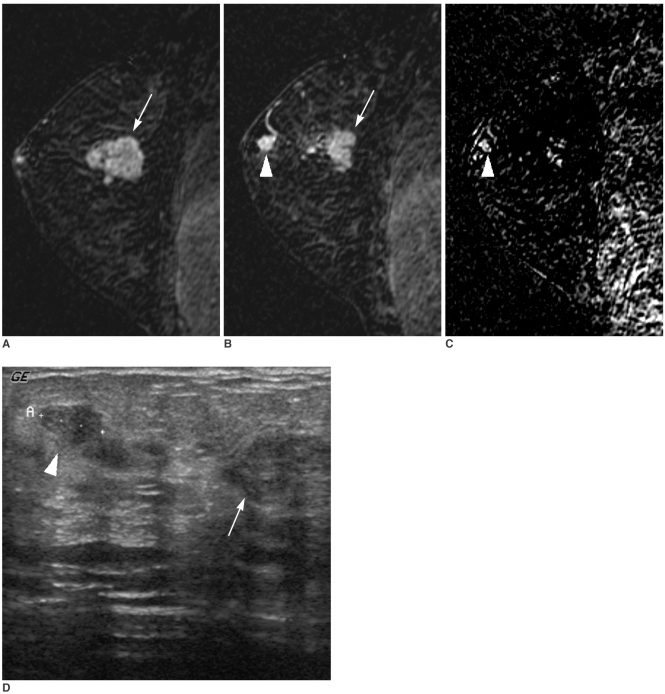
Fig. 2
A 56-year-old woman with a nonpalpable US-detected cancer.
A. A dynamic enhanced and subtracted T1-weighted sagittal MR image shows a spiculated malignant mass (arrow) in the upper outer portion of the right breast.
B, C. Multiple aggregated enhancing nodules (arrowhead) (B) are additionally noted in the lower outer portion of the right breast. These nodules (arrowhead) show a high signal intensity on the reverse subtracted image (C).
D. An initial US shows an irregular hypoechoic mass (arrow) in the upper outer portion of the right breast.
E. On targeted US, a hypoechoic nodule (arrowhead) that was not detected at an initial US is noted at the 9 o'clock position of the right breast. The surgical biopsy after US-guided localization revealed an intraductal papilloma.
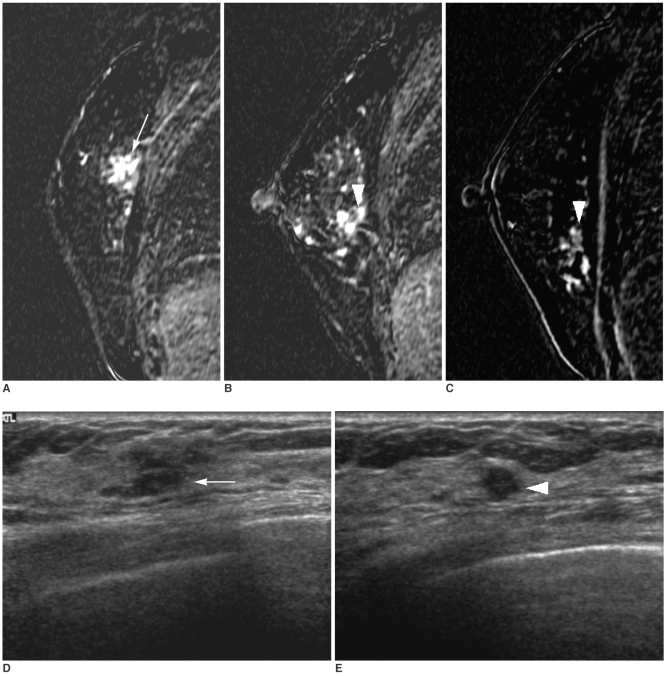
Fig. 3
A 40-year-old woman with a 1 cm palpable cancer of the left breast.
A, B. Dynamic enhanced and subtracted T1-weighted sagittal MR images show a 1 cm intensely enhancing main mass (arrow) (A) in addition to a 5 cm clumped segmental enhancement with washout (arrowheads) (B) in a different plane.
C. A high signal intensity (arrowheads) of a 5 cm clumped segmental enhancement is noted on the reverse subtracted image.
D. Initial US shows only a 1 cm hypoechoic mass (arrow) at a palpable site.
E. Targeted US shows a normal-looking, but slightly heterogeneous parenchyma. We interpreted that the additional enhancing lesion on MR had no US correlate. On a frozen section examination during the surgical operation, the additional enhancing lesion at the surrounding parenchyma revealed a malignancy, which was confirmed as an extensive intraductal carcinoma with an invasive ductal carcinoma following mastectomy. The surgical plan was changed from conservative breast surgery to mastectomy without the consent of the patient.
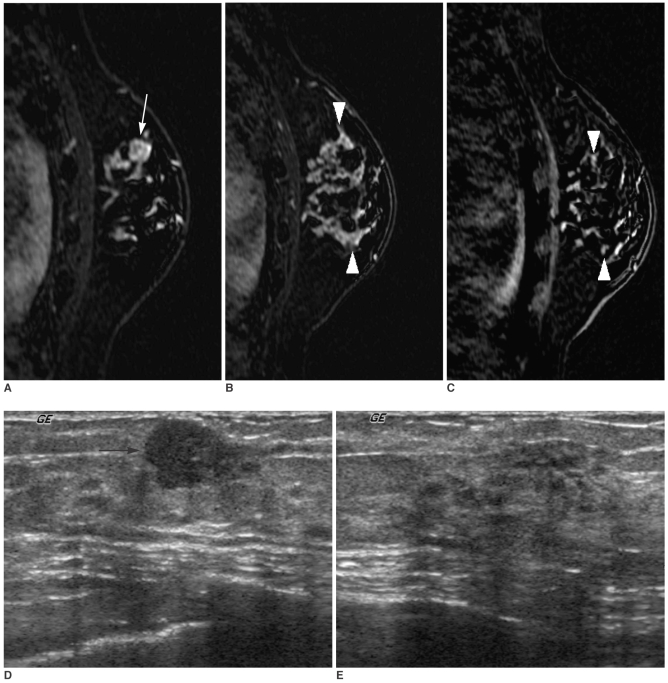
Fig. 4
A 43-year-old woman with a 1.5 cm palpable breast cancer.
A. A dynamic enhanced and subtracted T1-weighted sagittal MR image shows a 1.8 cm, irregular-shaped, enhancing mass (arrow) at the upper outer portion of the left breast.
B. Two additional suspicious enhancing lesions with washout (arrowheads) were noted at a different quadrant. Targeted US could not find a correlated lesion. Additional tissue excision and conserving surgery were performed due to this false positive finding of no US correlate. The final pathological diagnosis was fibrocystic change.
C. High signal intensities of two additional lesions (arrowheads) are noted on the reverse subtracted image.
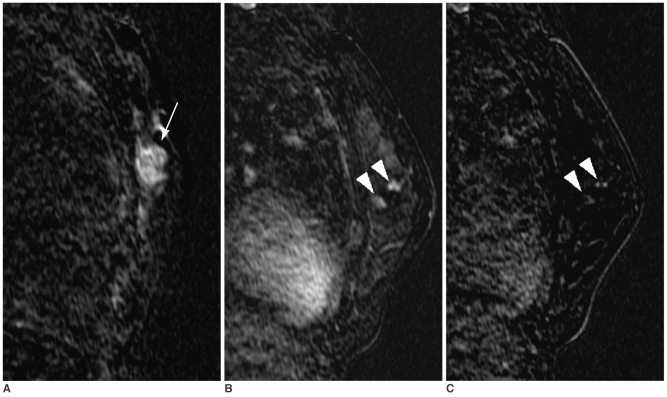
Table 1
Pathologic Outcomes of Additional Suspicious Lesions (n = 38) with the Performance of Targeted US in 31 Patients with Breast Cancer




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download