Abstract
Here, we report a case of scalp pseudoaneurysm which was treated by direct puncture embolization using n-butyl-2-cyanoacrylate. The patient had a history of blunt trauma in the previous two months. Ultrasound-guided manual compression was initially attempted, but the results were unsatisfactory. Direct puncture embolization was then performed, and the pseudoaneurysm was completely obliterated. Non-surgical treatment options for pseudoaneurysm are briefly discussed.
Pseudoaneurysms have classically been repaired by surgery. Recently, however, several alternatives have become available, and have been used due to the invasiveness of surgical repair (1, 2). These alternatives include US-guided compression and direct thrombin injections.
In this article, we report a case of posttraumatic scalp pseudoaneurysm, which was treated by direct puncture embolization, using n-butyl-2-cyanoacrylate (NBCA; B. Braun, Melsungen, Germany). US-guided manual compression was initially attempted, but the results were unsatisfactory. Non-surgical treatment options for pseudoaneurysm are also briefly discussed.
An 85-year-old female patient visited our hospital, complaining chiefly of scalp swelling. Two weeks earlier, the patient had fallen backward. Upon physical examination, we detected a subcutaneous mass in the patient's left posterior parietal area. The 3.5 cm mass was covered by normal skin. She also complained of a little pain on palpation. No tactile pulsation was detected. It was believed to be a posttraumatic subcutaneous hematoma, so we decided to continue observation, and wait for any spontaneous disappearance. The swelling in the patient's scalp decreased in size after one week. She attended no follow-up sessions thereafter.
Two months after her first visit, the patient was admitted to the emergency room due to scalp bleeding. The swelling in the patient's scalp had, at that time, increased to 4 cm in diameter with an overlying scalp defect which was oozing blood. Tactile pulsation was detected. The defect was sutured, and the bleeding was controlled via manual compression.
The patient was admitted for further evaluation of the pulsatile scalp mass. Brain CT revealed a slight increase in the attenuation of the hematoma in the left parietal scalp, with dense contrast filling (Fig. 1A). Upon routine laboratory examination, the level of creatinine in the patient's blood was higher than normal (22 mg/l, normal range 7-14).
Angiography of the external carotid artery revealed a pseudoaneurysm, which was fed by the left occipital artery (Fig. 1B). An aortogram revealed complete occlusion of the right renal artery, as well as severe proximal stenosis in the left renal artery. The radiologist (Y.H.C.) attempted to occlude the pseudoaneurysm via transarterial embolization. However, this attempt failed, due to the complex geometry of the proximal artery. A two-hour attempt was made to occlude the pseudoaneurysm via US-guided manual compression. Thereafter, the pulsation of the scalp swelling became negligible.
Doppler US, taken five days after the procedure, revealed thrombus formation in the pseudoaneurysm sac. We detected a residual blood flow to the inferior portion of the sac (Fig. 2).
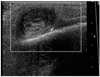
The patient's scalp swelling again developed pulsation two days after the taking of the ultrasound. At this time, an attempt was made to occlude the pseudoaneurysm via direct puncture embolization (Fig. 3). The pseudoaneurysm was completely occluded by treatment with a 1:1 mixture of NBCA and iodized oil (Lipiodol; Laboratoire Guerbet, Roissy, France).
No regrowth of the scalp swelling or pulsation occurred. Complete occlusion was confirmed by Doppler US, which was taken eight days later (Fig. 4). The scalp swelling subsided, and the wound eventually completely healed. There was no regrowth of the scalp pseudoaneurysm on follow-up for six months after direct puncture embolization.
In the head and neck, pseudoaneurysms are much more likely to be the result of trauma, although the majority of pseudoaneurysms are iatrogenic (3). The most frequent site of involvement is the superficial temporal artery, probably due to its location, as it is not protected by muscle (4). Pseudoaneurysms are produced by the partial transection of the arterial wall, and subsequent fibrous capsulation around a hematoma (5).
This case illustrates the efficacy of direct puncture embolization using NBCA as an alternative to surgical repair for pseudoaneurysms. Pseudoaneurysms are usually managed by US-guided manual compression (6). Manual compression may constitute a viable alternative to surgical repair, in cases in which the pseudoaneurysm is relatively small. Although surgery is the traditional treatment modality for scalp lesions (3), direct thrombin injections have been recently employed, due to the demonstrated usefulness of the technique. The thrombin injection technique was adopted subsequent to previous successes in the treatment of femoral pseudoaneurysms (7). However, there have also been reports of complications associated with the use of thrombin. This technique must be used cautiously for the treatment of pseudoaneurysms in arteries supplying vital end organs, such as those involving the extracranial circulation (8).
Although the pseudonaueurysm in this case could have been treated by surgical removal under local anesthesia, direct puncture embolization was performed as a second-line treatment. The reasons for our selection of nonoperative treatment are as follows: with direct puncture embolization, the problem can be resolved without incising the skin. Considering the actual size of the pseudoanueyrsm (diameter of about 1 cm), post-embolization shrinkage, and its location in the occipital area, we surmised that the cosmetic results would be acceptable. Repeated manual compressions were not attempted, as the results, despite two hours of manual compression, proved unsatisfactory.
In this case, we considered the use of direct thrombin injection after incomplete thrombus formation in US-guided compression. After directly puncturing the pseudoaneurysm, several test injections with liquid contrast media into the pseudoaneurysm under fluoroscopy revealed considerable reflux flow to the occipital artery. This finding led us to use NBCA, rather than thrombin. We believed that NBCA would cast the pseudoaneurysm more quickly than would thrombin, and that we would be able to control the injection amount relatively precisely, as we mixed NBCA with Lipiodol, for radiopacity during the injection. This allowed us to ascertain the appropriate time for the termination of the NBCA injection (9).
In pseudoaneurysms with short or wide necks, the use of thrombin may induce embolism as a complication. We suggest that NBCA may constitute a viable alternative to thrombin as, as mentioned above, it results in rapid casting, thus making it easier to terminate the injection at the appropriate time.
Figures and Tables
Fig. 1
Enhanced CT scan (A) and left occipital arteriogram (B) reveal a pseudoaneurysm in the scalp.
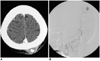
Fig. 2
Doppler ultrasonogram five days after manual compression reveals a crescent-shaped residual blood flow.
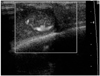
Fig. 3
Direct puncture embolization of pseudoaneurysm.
Test injection of contrast media after puncture (A) reveals reflux flow to the proximal occipital artery, in spite of cautious injection. Manual compression of the proximal occipital artery greatly reduces the extent of the reflux (B). After injection of a mixture of N-butyl-2-cyanoacrylate and Lipiodol during the manual compression of the proximal occipital artery, the cast-filling pseudoaneurysm is easily visualized on the fluoroscope (C).

References
1. Kang SS, Labropoulos N, Mansour MA, Baker WH. Percutaneous ultrasound guided thrombin injection: a new method for treating postcatheterization femoral pseudoaneurysms. J Vasc Surg. 1998. 27:1032–1038.
2. Paulson EK, Sheafor DH, Kliewer MA, Nelson RC, Eisenberg LB, Sebastian MW, et al. Treatment of iatrogenic femoral arterial pseudoaneurysms: comparison of US-guided thrombin injection with compression repair. Radiology. 2000. 215:403–408.
3. Isaacson G, Kochan PS, Kochan JP. Pseudoaneurysms of the superficial temporal artery: treatment options. Laryngoscope. 2004. 114:1000–1004.
4. Conner WC 3rd, Rohrich RJ, Pollock RA. Traumatic aneurysms of the face and temple: a patient report and literature review, 1644 to 1998. Ann Plast Surg. 1998. 41:321–326.
5. Cadamy AJ, McNaughton GW, Helliwell R. Traumatic pseudoaneurysms of the superficial temporal artery. Eur J Emerg Med. 2003. 10:236–237.
6. Paulson EK, Kliewer MA, Hertzberg BS, Tcheng JE, McCann RL, Bowie JD, et al. Ultrasonographically guided manual compression of femoral artery injuries. J Ultrasound Med. 1995. 14:653–659.
7. Partap VA, Cassoff J, Glikstein R. US-guided percutaneous thrombin injection: a new method of repair of superficial temporal artery pseudoaneurysm. J Vasc Interv Radiol. 2000. 11:461–463.
8. Teh LG, Sieunarine K. Thrombin injection for repair of pseudoaneurysms: a case for caution. Australas Radiol. 2003. 47:64–66.
9. Choi YH, Han MH, O-Ki K, Cha SH, Chang KH. Craniofacial cavernous venous malformations: percutaneous sclerotherapy with use of ethanolamine oleate. J Vasc Interv Radiol. 2002. 13:475–482.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download