Abstract
Women who have been treated for breast cancer are at risk for second breast cancers, such as ipsilateral recurrence or contralateral metachronous breast cancer. As the number of breast cancer survivors increases, interest in patient management and surveillance after treatment has also increased. However, post-treatment surveillance programs for patients with breast cancer have not been firmly established. In this review, we focus on the imaging modalities that have been used in post-treatment surveillance for patients with breast cancer, such as mammography, ultrasonography, magnetic resonance imaging, and positron emission tomography, the effectiveness of each modality for detecting recurrence, and how they can be applied to manage patients.
Breast cancer is one of the leading causes of death in women worldwide (1, 2) and is the second most common malignancy newly diagnosed in Korean women. More than 15000 women were newly diagnosed with breast cancer in 2011, and breast cancer was in second (21.5%) place among all newly diagnosed cancers in Korean women (www.ncc.re.kr). Moreover, the 5-year survival rate of breast cancer has increased from 83.2% to 91.3% over the last 10 years due to advances in postoperative treatment modalities and medications. The overall incidence of breast cancer in Korean women has increased from 1999 to 2011, and the prevalence of breast cancer during this period is 19.3% of all cancers in women (3), which agrees with reports on the Western population (1, 4). As the number of breast cancer survivors increases, patient management and surveillance after primary treatment has come under the spotlight. Women who have been treated for breast cancer are at risk for second breast cancers, such as tumor recurrence in the ipsilateral breast or a newly developed cancer in the contralateral breast (2, 4, 5). Reported risks for locoregional tumor recurrence range from 5-27%, whereas the risk for development of contralateral breast cancer is 5-10%, with a two six-fold increased risk (4, 6, 7, 8, 9, 10). In addition, recent studies have demonstrated that local recurrence is an independent predictor of survival, an high relative risks for developing distant metastases or breast cancer-related deaths in patients with local recurrences have been shown when compared to patients without a recurrence (10).
Considering these risks, a well-designed, evidence-based post-treatment surveillance protocol is needed to manage patients with breast cancer after their primary treatment. The surveillance program would be intended to detect second breast cancers at an early stage when curative intervention is possible. Up to now, mammography has been the only evidence-based imaging modality with demonstrated efficiency for detecting asymptomatic tumor recurrence or a second breast cancer in women who have been treated for primary breast cancer (2, 4, 11, 12, 13, 14, 15, 16, 17). Ultrasonography (US), magnetic resonance imaging (MRI) and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) have been utilized in many institutions to increase detection of second cancers at an early stage.
Screening mammography for women with an average risk of breast cancer results in early detection of breast cancer, leading to reduced mortality and improved patient outcome. Many case-controlled or non-randomized controlled trial (RCT) studies show a 20-30% reduction in breast cancer mortality after screening (18, 19). Hence, we assume that women with an elevated risk for breast cancer, including those who have already been treated for primary breast cancer, may benefit even more from screening mammography.
At present, mammography is the only imaging modality commonly recommended for breast cancer surveillance (Table 1) (1, 11, 20). As mammography enables detection of an early asymptomatic recurrence, early intervention or treatment is also possible (Fig. 1) (9, 21, 22). Several recent studies have demonstrated that early detection of a recurrence in asymptomatic patients during post-treatment follow-up improves survival (4, 22, 23), supporting the role of routine mammography for post-treatment surveillance of breast cancer. Based on a literature review, Houssami and Ciatto (4) reported that the proportion of ipsilateral breast recurrences detected on mammography is 50-80%, and mammography detects 45-90% of contralateral metachronous breast cancers. Paszat et al. (22) reported that surveillance mammography is associated with a significant reduction in the hazard for death related to breast cancer. Similarly, surveillance mammography helps detect asymptomatic tumor recurrence, resulting in improved patient survival, but most recommendations are based on consensus rather than evidence supported by RCTs. In another study, the proportion of ipsilateral breast recurrences detected with mammography was 8-51% of lesions detected on mammography only, but approximately three-fifths of the participating hospitals perform mammography surveillance at 6-month intervals for 2 to 5 years in patients with breast conservation surgery (24). Such semiannual mammographic surveillance allows the detection of a significantly higher proportion of cancer recurrences at an earlier stage than that of annual surveillance (25). However, these results do not support establishing intensive surveillance because no significant differences was found in tumor size or nodal status between the semiannual surveillance and the annual surveillance groups, and the follow-up intervals were 3-18 months, which was too long to strictly separate the patients into two groups (26). As seen in the various reports mentioned above, although most studies include mammography for post-treatment surveillance of women who have been treated for breast cancer, two important issues remain unsolved. The mammography follow-up interval and the follow-up duration need to be defined. The more popular post-treatment surveillance recommendations for patients with breast cancer are summarized in Table 1. The American Society of Clinical Oncology Clinical Practice Guidelines recommend a post-treatment mammogram 1 year after initial diagnosis or at least 6 months after completion of radiation therapy, and yearly mammography follow-up thereafter (11). Similarly, the National Comprehensive Cancer Network (www.nccn.org, version 2013.03) and the National Institute for Clinical Excellence guidelines (www.nice.org.uk, 2011) recommend mammography every 12 months in addition to routine history and physical examinations obtained at regular visits. As in the prior recommendations, we agree that annual mammography should be performed for 5 years after treatment, and a mammography every 1-2 years thereafter may be a reasonable compromise.
It should be emphasized that there is insufficient evidence regarding mammography follow-up intervals in post-treatment surveillance. Additionally, quantifying the actual impact of screening mammography in these patients excluding bias, specifically lead-time and length-time bias, is difficult using mostly non-randomized retrospective studies. RCTs are the most appropriate method to estimate the effect of early detection of ipsilateral or contralateral breast cancer recurrence, but applying this study design to clinical practice is not feasible or ethical, as patients who have been treated for breast cancer are already at high risk for developing second breast cancers. Further prospective investigation that includes a large data set showing how women with a personal breast cancer history benefit from surveillance mammography is anticipated in the future, along with evidence-based meticulous screening programs that will build on the results.
Ultrasonography is a widely available, relatively inexpensive imaging method that is easy to perform, has no radiation hazards, does not require a contrast agent, and enables biopsy under image guidance. Breast US has been popularly used to characterize lesions and differentially diagnose breast masses as an adjunctive tool to mammography, particularly in women with dense breasts (5, 27). Preoperative bilateral whole-breast US also provides complementary information to mammography (28, 29), and detects up to 88% of contralateral synchronous cancers, among which 43% are occult on a mammogram (28). Based on the results of preoperative US, one study showed that approximately 16% of women who had undergone preoperative US had changes in treatment plans set by mammography alone (29).
Moreover, US has drawn attention as a useful surveillance imaging method in addition to mammography in women who have been treated for breast cancer. US detects ipsilateral recurrent or contralateral metachronous breast cancers with higher sensitivity (91-97%) (Table 2) than that of palpation or mammography, which have sensitivity values of 45.5-79% and 45-87%, respectively (5, 8, 28, 30, 31, 32). Adding US to mammography in the American College of Radiology Imaging Network (ACRIN) 6666 trials yielded an additional 1.1-7.2 cancers per 1000 high-risk women, of which 53% of the 2637 enrolled women had a personal history of breast cancer (27). Other than the breast, US is an excellent modality to evaluate chest wall and axillary areas, which cannot be easily approached by mammography. One of the most common sites for post-treatment breast cancer recurrence is the chest wall (Fig. 2), either from direct extension of the tumor, indirect extension via interpectoral nodes, or from undissected lymphatics (32). Approximately 10-35% of patients who have been treated for breast cancer have a metastasis in the axillary, internal mammary, and supraclavicular nodes (33, 34). Among the occult regional recurrences after surgical treatment for breast cancer, only 21.4% occur at the axillary area lateral to the pectoralis muscle, which may be included within the fields of mammography and surgical sampling, while the remaining 78.6% of regional recurrence was detected by US alone at areas other than the axilla (34, 35, 36). In other studies, the sensitivity of mammography is only 10% (0.0-14.3%), whereas that of US was around 90% (81.1-100.0%) to detect regional or locoregional recurrences (24, 34, 37, 38, 39, 40).
Several studies have shown surveillance results of US applied to women who were treated for breast cancer (34, 37, 39, 40, 41). The reported cancer detection rates were 1.7-5.1% per patient, and the positive predictive value (PPV) was 21.5-52.6%, with percentages varying according to the area involved. Because isolated recurrences are associated with distant metastasis and/or poor outcome, early detection and targeted treatment for recurrences are critical to improve patient outcome (41, 42, 43). Early detection of a locoregional recurrence of breast cancer after primary treatment by US can help guide patient management by sorting out those who may benefit by early therapeutic intervention or curative treatment of local disease. Patients who received a mastectomy and who had a locoregional recurrence detected at an early stage or an isolated regional recurrence had better survival with short follow-up (23, 29, 41), but controversy remains about whether post-treatment surveillance US actually affects long term survival in patients with breast cancer (40) and whether all asymptomatic regional recurrences will be detected by US or by PET-CT or another imaging modality in clinical practice (42).
One important point to discuss is which patients should be recommended for US surveillance. Many studies agree that mammography is the basic imaging modality for breast cancer surveillance (5), and the usefulness of US surveillance can be amplified under conditions in which the benefits of mammographic surveillance are reduced such as in dense breasts. The sensitivity of US for detecting metachronous breast cancer is 94%, regardless of breast density, whereas sensitivity of mammography in women with dense breasts is lower than that of women with scattered fibroglandular tissue (73% vs. 80%) (5). In contrast, results of another study show that the differences in sensitivity between mammography and US for non-palpable second breast cancers is not noticeably different for ipsilateral breast tumor recurrence (14%) compared to that of contralateral breast recurrence (28%), and that the sensitivity of US for non-palpable breast tumor recurrences drops by about 10% in the ipsilateral breast, which may due to sonographically architectural distortion obscuring detection of an ipsilateral breast tumor recurrence (38). Additionally, the main shortcoming of US in the ACRIN 6666 trial was the substantial false-positive rate; the PPV of the biopsy recommendation after US examinations was < 10.0%. However, after reviewing other reports of investigators performing sequential US, the PPV was approximately 41% (18 of 44 lesions) in the contralateral breast (32) and 67% in bilateral breasts (34). As seen in the heterogeneous results from prior studies, although US may have its strong points for visualizing areas that cannot be approached by mammography or provide additional information regarding differentiation between postoperative changes and locoregional recurrence, little evidence suggests whether US is effective and beneficial for improving survival of patients with breast cancer and the role of US in post-treatment surveillance programs has yet to be investigated.
Breast MRI is an important supplementary imaging modality to conventional breast cancer evaluation methods, particularly for preoperative planning due to its high sensitivity (95-100%) in disease detection (44, 45, 46), among which the amount of glandular density does not have an influence. The reported sensitivity of breast MRI for detecting tumors in high risk women is significantly higher than that of mammography (77-100%), and specificity in an acceptable range of 81-95% (45, 46, 47). However, the women included in these studies were high risk, has a strong family history of breast cancer, or were suspected or tested to have breast cancer susceptibility genes, such as the BRCA mutations. Although breast MRI is recommended for screening in women with a lifetime breast cancer risk > 20-25% by the American Cancer Society (20), the American College of Radiology (47), and the Society of Breast Imaging (48), these recommendations only state that MRI "may be considered" for women who have a personal history of breast cancer; that is, women with intermediate risk (lifetime breast cancer risk > 15-20%) for breast cancer (47). Therefore, little is known about the role of breast MRI in post-treatment surveillance programs.
Breast MRI shows high sensitivity and specificity for differentiating between post-treatment changes in the breast from recurrent malignancies (Table 2) (31, 49), particularly when performed later than 12 months after treatment (50). In a study by Brennan et al. (49), breast screening with MRI detected cancer in 12% of the study population, including women with a personal history of breast cancer only, and the PPV of the biopsy recommendations from MRI was acceptable at 39%. Based on their results, the authors claimed that MRI screening of women with a personal history of breast cancer is clinically valuable because cancers discovered from screening MRI benefit from early detection, as more than half were minimal breast cancers (49). This and the results of another study by Morris et al. (51) emphasize the importance of a personal history of breast cancer treatment as an indication for MRI screening. The PPV of biopsy recommendations based on MRI features is 32% in women with a family history of breast cancer and it increases to 50% in women who have also been diagnosed and treated for breast cancer (51). Breast MRI screening has the highest yield in women with both a family and personal history of breast cancer, particularly in those who have had breast conservation surgery (51). Additionally, high negative predictive values of breast MRI have been reported in women who had breast conservation therapy; Belli et al. (31) concluded that the absence of enhancing foci in post-treatment breasts has 100% reliability for predicting the absence of tumor recurrence. There may be no need for additional invasive procedures such as biopsy if there are no enhancing foci by correlating lesions that are suspected to be recurrences on conventional imaging modalities to breast MRI. Patients may benefit from screening MRI, with its high NPV, by reducing many benign biopsies, which is supported by the results of another study (52) concluding that a negative MRI is more useful and conclusive than a positive MRI, as positive features warrant further investigation. This is also supported by a recent study that included a large proportion of women who had been treated for breast cancer with additionally detected early stage breast cancers on supplemental screening breast MRI performed in addition to mammography and US; 14.7 more cancers per 1000 women were additionally detected by MRI (25).
MRI for surveillance or screening purposes is limited, as this imaging method is expensive, lacks availability, requires contrast media injection for adequate imaging, and neither the technique nor interpretive criteria for breast MRI are standardized (51). Despite its shortcomings, breast MRI is a highly sensitive imaging modality. However, when and in which circumstances screening breast MRI should be applied is still a question for women who have been treated for breast cancer. As most postoperative women undergoing surveillance are under a hormonal therapy that suppresses the ovary, use of MRI is supported in postoperative surveillance programs. As in the many studies on mammography or US, studies evaluating the efficacy of MRI for post-treatment surveillance in women who have been treated for breast cancer are of retrospective design and include a limited number of patients. Therefore, further randomized prospective studies are needed to properly assess the role of breast MRI in post-treatment surveillance programs.
Positron emission tomography/CT has been used for preoperative staging and the treatment response of patients with breast cancer. This hybrid imaging method has a strong point as it enables anatomic localization of the PET signal via CT (53). PET/CT is particularly useful in patients who are suspected to have or who are exhibiting a recurrence on physical examination or conventional imaging methods (53, 54). Because PET/CT is highly sensitive for detecting lesions and determining whether a recurrence is solitary or disseminated, information gained from PET/CT has a significant impact on the decision for upcoming treatment and post-treatment patient outcome. Sensitivity of PET/CT for detecting locoregional recurrence or metastasis among patients with breast cancer is approximately 97%, with a diagnostic accuracy of 95% in one study (55), supporting the efficacy of PET/CT for patients diagnosed or suspected of having recurrent breast cancer (Fig. 3). However, there is a lack of evidence demonstrating the efficacy and cost-effectiveness of this modality, along with the hazard of radiation exposure and the absence of specific clinical indications (56).
Current post-treatment surveillance guidelines for patients with treated breast cancer do not recommend intensive surveillance, such as routine chest radiography, bone scans, or laboratory tests, to evaluate distant recurrence or metastatic diseases. Studies have reported prolonged survival in patients who had asymptomatic metastatic lesions detected at an early stage but early detection was not advantageous to patient survival when the time of initial breast cancer diagnosis was applied. In other words, lead-time bias or length-time bias may have misled thinking that these intensive surveillance programs may prolong patient survival (57). Nevertheless, post-treatment surveillance programs applied to patients with breast cancer vary among organizations and countries, mostly due to the lack of a standardized protocol, and studies evaluating distant metastasis are quite often performed along with mammography and physical examinations. A recent study based on the Texas Cancer Registry (56) represents the current status of imaging modalities in post-treatment surveillance programs; only 55.3% of patients treated for breast cancer showed strict adherence to the current surveillance program, including a routine physical examination and mammography. During the 2001-2007 study period, use of mammography and bone scans decreased (81% to 75% and 21% to 13%, respectively), whereas use of MRI and PET-CT increased significantly (0.5% to 7.0% and 2% to 9%, respectively) (56). Based on their report, it is evident that clinicians and patients do not feel that the current surveillance program with mammography as the only imaging modality is sufficient. Although evidence does not yet demonstrate that early detection of a distant metastasis improves patient survival, early detection provides a chance for curative intervention, which may affect quality of life or long-term survival of patients, and this may be the cause for the current trend in which supplemental surveillance imaging modalities other than just mammography are used. However, not all patients benefit from these extensive and rather costly studies repeated annually, and the accuracy of the additional imaging modalities has not yet been confirmed. Additionally, these additional modalities may reveal many false-positive lesions, particularly MRI and PET scans (20, 54, 55), and may provoke unnecessary interventional procedures and patient anxiety. Considering the development of new imaging technologies and the desire of clinicians and patients for better supplementary surveillance methods, it is difficult to insist on patient compliance with the current surveillance program. Therefore, discrete evidence on the cost-effectiveness and accuracy of applying supplemental modalities to mammography in terms of patient survival is required when used for post-treatment surveillance of breast cancer.
Currently, mammography is the single imaging modality recommended for routine follow-up surveillance in women who have been treated for breast cancer. The role of additional imaging modalities, such as US, MRI, and PET-CT, as post-treatment surveillance in women treated for breast cancer has not yet been established, but they are potentially useful and show high sensitivity and accuracy for detecting recurrences or distant metastases. Although many studies have demonstrated the efficacy of these additional imaging modalities when applied to post-treatment surveillance, they are currently used in clinical practice without specific clinical indications or organized programs due to a lack of concrete evidence. An evaluation of the cost-effectiveness of these imaging modalities should be considered because of their additional costs. A number of different guidelines regarding post-treatment surveillance of patients with breast cancer have been produced worldwide. This is mostly from efforts to ensure that patients should undergo the most appropriate follow-up to decrease patient morbidity and mortality and enable long-term survival after treatment. The heterogeneity regarding post-treatment patient management may arise from the lack of solid evidence on the potential benefits of each follow-up imaging modality. The multiplicity of guidelines or recommendations may reflect that socioeconomic conditions, mostly financial causes such as insurance policies, vary among countries and institutions. This is an important matter that should be considered when investigating the most appropriate and effective method for post-treatment surveillance of patients with breast cancer. Further prospective studies including a large number of patients are expected in the future to demonstrate the role of various imaging modalities in post-treatment surveillance programs and how they affect survival in patients treated for breast cancer.
References
1. Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24(Suppl 6):vi7–vi23. PMID: 23970019.

2. Houssami N, Ciatto S, Martinelli F, Bonardi R, Duffy SW. Early detection of second breast cancers improves prognosis in breast cancer survivors. Ann Oncol. 2009; 20:1505–1510. PMID: 19297316.

3. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014; 46:109–123. PMID: 24851102.

4. Houssami N, Ciatto S. Mammographic surveillance in women with a personal history of breast cancer: how accurate? How effective? Breast. 2010; 19:439–445. PMID: 20547457.

5. Kim MJ, Kim EK, Kwak JY, Park BW, Kim SI, Sohn J, et al. Role of sonography in the detection of contralateral metachronous breast cancer in an Asian population. AJR Am J Roentgenol. 2008; 190:476–480. PMID: 18212235.

6. Grunfeld E, Noorani H, McGahan L, Paszat L, Coyle D, van Walraven C, et al. Surveillance mammography after treatment of primary breast cancer: a systematic review. Breast. 2002; 11:228–235. PMID: 14965672.

7. Karam AK. Breast cancer posttreatment surveillance: diagnosis and management of recurrent disease. Clin Obstet Gynecol. 2011; 54:157–163. PMID: 21278515.

8. Rissanen TJ, Mäkäräinen HP, Mattila SI, Lindholm EL, Heikkinen MI, Kiviniemi HO. Breast cancer recurrence after mastectomy: diagnosis with mammography and US. Radiology. 1993; 188:463–467. PMID: 8327698.

9. Voogd AC, van Tienhoven G, Peterse HL, Crommelin MA, Rutgers EJ, van de Velde CJ, et al. Local recurrence after breast conservation therapy for early stage breast carcinoma: detection, treatment, and outcome in 266 patients. Dutch Study Group on Local Recurrence after Breast Conservation (BORST). Cancer. 1999; 85:437–446. PMID: 10023713.
10. Kemperman H, Borger J, Hart A, Peterse H, Bartelink H, van Dongen J. Prognostic factors for survival after breast conserving therapy for stage I and II breast cancer. The role of local recurrence. Eur J Cancer. 1995; 31A:690–698. PMID: 7640040.

11. Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013; 31:961–965. PMID: 23129741.

12. Ciatto S, Miccinesi G, Zappa M. Prognostic impact of the early detection of metachronous contralateral breast cancer. Eur J Cancer. 2004; 40:1496–1501. PMID: 15196532.

13. Lu W, Schaapveld M, Jansen L, Bagherzadegan E, Sahinovic MM, Baas PC, et al. The value of surveillance mammography of the contralateral breast in patients with a history of breast cancer. Eur J Cancer. 2009; 45:3000–3007. PMID: 19744851.

14. Robertson C, Ragupathy SK, Boachie C, Fraser C, Heys SD, Maclennan G, et al. Surveillance mammography for detecting ipsilateral breast tumour recurrence and metachronous contralateral breast cancer: a systematic review. Eur Radiol. 2011; 21:2484–2491. PMID: 21833567.

15. Rojas MP, Telaro E, Russo A, Moschetti I, Coe L, Fossati R, et al. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2005; CD001768. PMID: 15674884.

16. Hayes DF. Clinical practice. Follow-up of patients with early breast cancer. N Engl J Med. 2007; 356:2505–2513. PMID: 17568031.
17. Association of Breast Surgery @ BASO, Royal College of Surgeons of England. Guidelines for the management of symptomatic breast disease. Eur J Surg Oncol. 2005; 31(Suppl 1):1–21.
18. Gabe R, Duffy SW. Evaluation of service screening mammography in practice: the impact on breast cancer mortality. Ann Oncol. 2005; 16(Suppl 2):ii153–ii162. PMID: 15958448.

19. Roder D, Houssami N, Farshid G, Gill G, Luke C, Downey P, et al. Population screening and intensity of screening are associated with reduced breast cancer mortality: evidence of efficacy of mammography screening in Australia. Breast Cancer Res Treat. 2008; 108:409–416. PMID: 18351455.

20. Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007; 57:75–89. PMID: 17392385.

21. Montgomery DA, Krupa K, Jack WJ, Kerr GR, Kunkler IH, Thomas J, et al. Changing pattern of the detection of locoregional relapse in breast cancer: the Edinburgh experience. Br J Cancer. 2007; 96:1802–1807. PMID: 17533401.

22. Paszat L, Sutradhar R, Grunfeld E, Gainford C, Benk V, Bondy S, et al. Outcomes of surveillance mammography after treatment of primary breast cancer: a population-based case series. Breast Cancer Res Treat. 2009; 114:169–178. PMID: 18368477.

23. Lu WL, Jansen L, Post WJ, Bonnema J, Van de Velde JC, De Bock GH. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: a meta-analysis. Breast Cancer Res Treat. 2009; 114:403–412. PMID: 18421576.

24. Obdeijn IM, Winter-Warnars GA, Mann RM, Hooning MJ, Hunink MG, Tilanus-Linthorst MM. Should we screen BRCA1 mutation carriers only with MRI? A multicenter study. Breast Cancer Res Treat. 2014; 144:577–582. PMID: 24567197.

25. Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012; 307:1394–1404. PMID: 22474203.

26. Dershaw DD, Lee CH, Morris EA. Mammographic surveillance after breast conservation therapy. Radiology. 2013; 266:685. PMID: 23362098.

27. Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008; 299:2151–2163. PMID: 18477782.

28. Kim MJ, Kim EK, Kwak JY, Park BW, Kim SI, Oh KK. Bilateral synchronous breast cancer in an Asian population: mammographic and sonographic characteristics, detection methods, and staging. AJR Am J Roentgenol. 2008; 190:208–213. PMID: 18094313.

29. Moon WK, Noh DY, Im JG. Multifocal, multicentric, and contralateral breast cancers: bilateral whole-breast US in the preoperative evaluation of patients. Radiology. 2002; 224:569–576. PMID: 12147858.

30. Balu-Maestro C, Bruneton JN, Geoffray A, Chauvel C, Rogopoulos A, Bittman O. Ultrasonographic posttreatment follow-up of breast cancer patients. J Ultrasound Med. 1991; 10:1–7. PMID: 1997679.

31. Belli P, Costantini M, Romani M, Marano P, Pastore G. Magnetic resonance imaging in breast cancer recurrence. Breast Cancer Res Treat. 2002; 73:223–235. PMID: 12160328.

32. Kim SM, Park JM. Normal and abnormal US findings at the mastectomy site. Radiographics. 2004; 24:357–365. PMID: 15026586.

33. Kim MJ, Kim EK, Kwak JY, Park BW, Kim SI, Sohn J, et al. Sonographic surveillance for the detection of contralateral metachronous breast cancer in an Asian population. AJR Am J Roentgenol. 2009; 192:221–228. PMID: 19098203.

34. Shin JH, Han BK, Choe YH, Nam SJ, Park W, Im YH. Ultrasonographic detection of occult cancer in patients after surgical therapy for breast cancer. J Ultrasound Med. 2005; 24:643–649. PMID: 15840796.

35. Eubank WB, Mankoff DA, Vesselle HJ, Eary JF, Schubert EK, Dunnwald LK, et al. Detection of locoregional and distant recurrences in breast cancer patients by using FDG PET. Radiographics. 2002; 22:5–17. PMID: 11796893.

36. Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, Jhingran A, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000; 18:2817–2827. PMID: 10920129.

37. Kanso H, Kazzi H, Menassa-Moussa L, Abi Khalil S, Ghosn M, Chahine G, et al. [Value of US imaging following mastectomy]. J Radiol. 2008; 89(9 Pt 1):1077–1080. PMID: 18772785.
38. Kim SJ, Moon WK, Cho N, Chang JM. The detection of recurrent breast cancer in patients with a history of breast cancer surgery: comparison of clinical breast examination, mammography and ultrasonography. Acta Radiol. 2011; 52:15–20. PMID: 21498320.

39. Lee JH, Kim EK, Oh JY, Kwon HC, Kim SH, Kim DC, et al. US screening for detection of nonpalpable locoregional recurrence after mastectomy. Eur J Radiol. 2013; 82:485–489. PMID: 23131395.

40. Suh YJ, Kim MJ, Kim EK, Moon HJ, Kim SI, Park BW. Value of ultrasound for postoperative surveillance of asian patients with history of breast cancer surgery: a single-center study. Ann Surg Oncol. 2013; 20:3461–3468. PMID: 23695431.

41. Kim HJ, Kwak JY, Choi JW, Bae JH, Shin KM, Lee HJ, et al. Impact of US surveillance on detection of clinically occult locoregional recurrence after mastectomy for breast cancer. Ann Surg Oncol. 2010; 17:2670–2676. PMID: 20422455.

42. Moon HJ, Kim MJ, Kim EK, Park BW, Youk JH, Kwak JY, et al. US surveillance of regional lymph node recurrence after breast cancer surgery. Radiology. 2009; 252:673–681. PMID: 19546429.

43. Schmoor C, Sauerbrei W, Bastert G, Schumacher M. Role of isolated locoregional recurrence of breast cancer: results of four prospective studies. J Clin Oncol. 2000; 18:1696–1708. PMID: 10764430.

44. Boetes C, Veltman J. Screening women at increased risk with MRI. Cancer Imaging. 2005; 5(Spec No A):S10–S15. PMID: 16361123.

45. Echevarria JJ, Martín M, Saiz A, Imaz I, Férnandez-Ruanova B, Martín D, et al. Overall breast density in MR mammography: diagnostic and therapeutic implications in breast cancer. J Comput Assist Tomogr. 2006; 30:140–147. PMID: 16365590.
46. Lee SG, Orel SG, Woo IJ, Cruz-Jove E, Putt ME, Solin LJ, et al. MR imaging screening of the contralateral breast in patients with newly diagnosed breast cancer: preliminary results. Radiology. 2003; 226:773–778. PMID: 12601182.

47. Mainiero MB, Lourenco A, Mahoney MC, Newell MS, Bailey L, Barke LD, et al. ACR Appropriateness Criteria Breast Cancer Screening. J Am Coll Radiol. 2013; 10:11–14. PMID: 23290667.

48. Lee CH, Dershaw DD, Kopans D, Evans P, Monsees B, Monticciolo D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010; 7:18–27. PMID: 20129267.

49. Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010; 195:510–516. PMID: 20651211.

50. Viehweg P, Heinig A, Lampe D, Buchmann J, Heywang-Köbrunner SH. Retrospective analysis for evaluation of the value of contrast-enhanced MRI in patients treated with breast conservative therapy. MAGMA. 1998; 7:141–152. PMID: 10050940.

51. Morris EA, Liberman L, Ballon DJ, Robson M, Abramson AF, Heerdt A, et al. MRI of occult breast carcinoma in a high-risk population. AJR Am J Roentgenol. 2003; 181:619–626. PMID: 12933450.

52. Quinn EM, Coveney AP, Redmond HP. Use of magnetic resonance imaging in detection of breast cancer recurrence: a systematic review. Ann Surg Oncol. 2012; 19:3035–3041. PMID: 22476755.

53. Groheux D, Espié M, Giacchetti S, Hindié E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013; 266:388–405. PMID: 23220901.

54. Pan L, Han Y, Sun X, Liu J, Gang H. FDG-PET and other imaging modalities for the evaluation of breast cancer recurrence and metastases: a meta-analysis. J Cancer Res Clin Oncol. 2010; 136:1007–1022. PMID: 20091186.

55. Aukema TS, Rutgers EJ, Vogel WV, Teertstra HJ, Oldenburg HS, Vrancken Peeters MT, et al. The role of FDG PET/CT in patients with locoregional breast cancer recurrence: a comparison to conventional imaging techniques. Eur J Surg Oncol. 2010; 36:387–392. PMID: 19962268.

56. Parmar AD, Sheffield KM, Vargas GM, Han Y, Chao C, Riall TS. Quality of post-treatment surveillance of early stage breast cancer in Texas. Surgery. 2013; 154:214–225. PMID: 23889950.

57. Rosselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA. 1994; 271:1593–1597. PMID: 7848404.

58. Drew PJ, Kerin MJ, Turnbull LW, Imrie M, Carleton PJ, Fox JN, et al. Routine screening for local recurrence following breast-conserving therapy for cancer with dynamic contrast-enhanced magnetic resonance imaging of the breast. Ann Surg Oncol. 1998; 5:265–270. PMID: 9607630.

Fig. 1
41-year-old woman who had undergone right partial mastectomy due to invasive ductal carcinoma.
Follow-up mammography (A) performed 26 months after surgery revealed mass (arrows) at mastectomy site, which was more prominent compared to follow-up mammography performed 6 months before. Ultrasonography (B) showed 15-mm mass in right upper outer breast correlating to mass detected on mammography. Breast magnetic resonance imaging (C) showed peripherally enhanced mass in right breast (arrows). Subsequent biopsy and surgery were performed and revealed invasive ductal carcinoma.
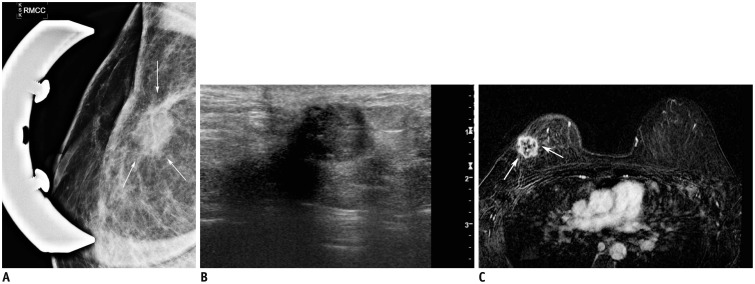
Fig. 2
44-year-old woman who had undergone modified radical mastectomy of left breast due to invasive ductal carcinoma.
Ultrasonography (US) performed 30 months after surgery (A) revealed 11-mm hypoechoic lesion located within skin layer (arrow). US-guided fine needle aspiration was performed on this lesion, and cytology result was positive for metastatic carcinoma from breast. Breast magnetic resonance imaging (B) showed enhanced nodule in left chest wall (arrow) correlating to proven malignant mass.
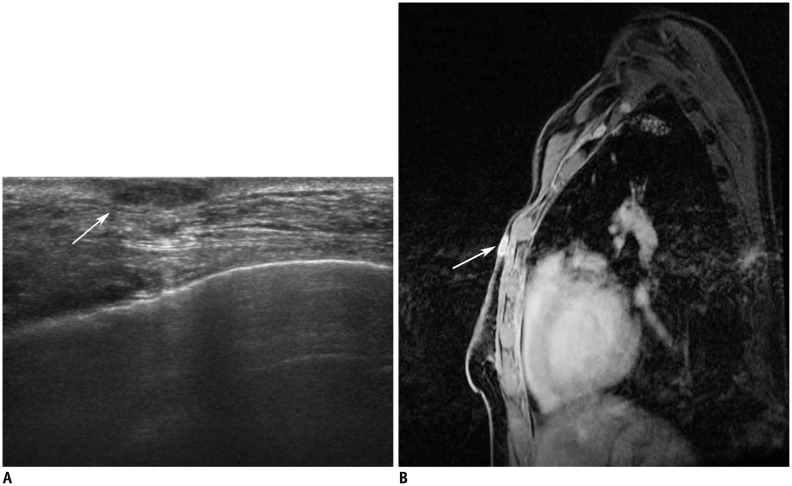
Fig. 3
63-year-old woman who had undergone left mastectomy due to invasive ductal carcinoma.
Negative findings were seen on follow-up mammography and ultrasonography performed for surveillance. Follow-up positron emission tomography-computed tomography scan (A, arrows) performed 38 months later for surveillance revealed multiple areas of increased fluorodeoxyglucose uptake in both lungs, mediastinum, and liver. CT scans revealed multiple metastatic nodules in both lower lungs (B, arrows), enlarged metastatic mediastinal lymph nodes (C, arrows), and low-attenuating metastatic mass in caudate lobe of liver (D, arrow).
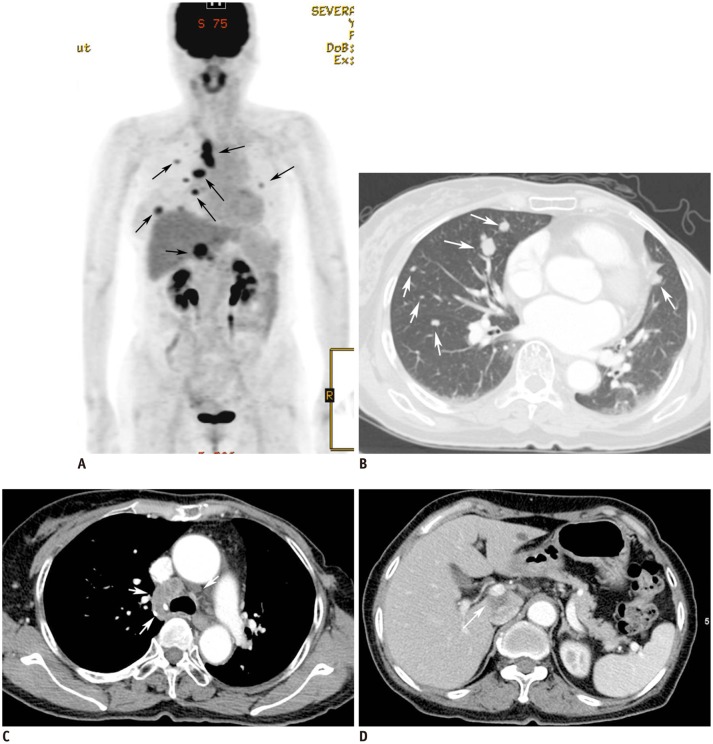
Table 1
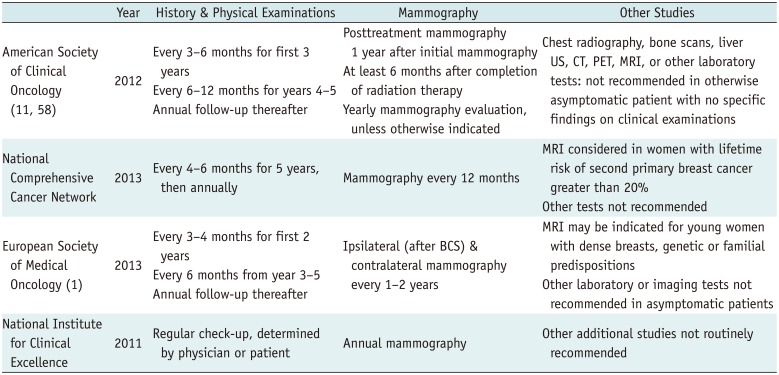
Post-Treatment Surveillance Recommendations for Women Treated for Primary Breast Cancer
| Year | History & Physical Examinations | Mammography | Other Studies | |
|---|---|---|---|---|
| American Society of Clinical Oncology (11, 58) | 2012 | Every 3-6 months for first 3 years | Posttreatment mammography 1 year after initial mammography | Chest radiography, bone scans, liver US, CT, PET, MRI, or other laboratory tests: not recommended in otherwise asymptomatic patient with no specific findings on clinical examinations |
| Every 6-12 months for years 4-5 Annual follow-up thereafter | At least 6 months after completion of radiation therapy | |||
| Yearly mammography evaluation, unless otherwise indicated | ||||
| National Comprehensive Cancer Network | 2013 | Every 4-6 months for 5 years, then annually | Mammography every 12 months | MRI considered in women with lifetime risk of second primary breast cancer greater than 20% |
| Other tests not recommended | ||||
| European Society of Medical Oncology (1) | 2013 | Every 3-4 months for first 2 years | Ipsilateral (after BCS) & contralateral mammography every 1-2 years | MRI may be indicated for young women with dense breasts, genetic or familial predispositions |
| Every 6 months from year 3-5 Annual follow-up thereafter | Other laboratory or imaging tests not recommended in asymptomatic patients | |||
| National Institute for Clinical Excellence | 2011 | Regular check-up, determined by physician or patient | Annual mammography | Other additional studies not routinely recommended |
Table 2
Diagnostic Performances of Mammography, Ultrasonography, and MRI in Post-Treatment Surveillance of Breast Cancer Patients
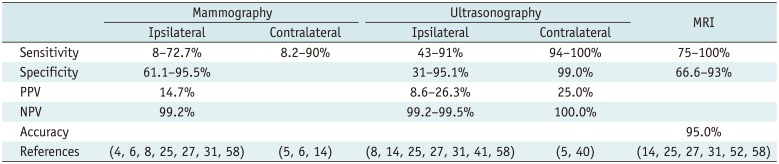
| Mammography | Ultrasonography | MRI | |||
|---|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | ||
| Sensitivity | 8-72.7% | 8.2-90% | 43-91% | 94-100% | 75-100% |
| Specificity | 61.1-95.5% | 31-95.1% | 99.0% | 66.6-93% | |
| PPV | 14.7% | 8.6-26.3% | 25.0% | ||
| NPV | 99.2% | 99.2-99.5% | 100.0% | ||
| Accuracy | 95.0% | ||||
| References | (4, 6, 8, 25, 27, 31, 58) | (5, 6, 14) | (8, 14, 25, 27, 31, 41, 58) | (5, 40) | (14, 25, 27, 31, 52, 58) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download