This article has been corrected. See "Erratum" in Volume 13 on page 256.
Abstract
Objective
To compare the iterative decomposition of water and fat with echo asymmetry and the least-squares estimation (IDEAL) method with a fat-saturated T2-weighted (T2W) fast recovery fast spin-echo (FRFSE) imaging of the spine.
Materials and Methods
Images acquired at 3.0 Tesla (T) in 35 patients with different spine lesions using fat-saturated T2W FRFSE imaging were compared with T2W IDEAL FRFSE images. Signal-to-noise ratio (SNR)-efficiencies measurements were made in the vertebral bodies and spinal cord in the mid-sagittal plane or nearest to the mid-sagittal plane. Images were scored with the consensus of two experienced radiologists on a four-point grading scale for fat suppression and overall image quality. Statistical analysis of SNR-efficiency, fat suppression and image quality scores was performed with a paired Student's t test and Wilcoxon's signed rank test.
Over the past two decades, MR imaging has evolved into the preferred imaging modality for evaluating pathology of the vertebrae and soft tissue structures within and adjacent to the spine (1). The normal spine contains red marrow rich in adipocytes, and as the patient ages, fat content increases progressively (2-4). Meanwhile, abundant adipose tissues exist in perispinal structures (2, 5). Fat in the marrow and perispinal structures appears bright on fast spin-echo (FSE) T1-weighted (T1W) and T2-weighted (T2W) images. Furthermore, a variety of pathologies usually appear in a high signal intensity similar to fat signal on T2W images (1, 2, 6-8). Therefore, the normal fat may obscure underlying pathologies that can involve the spine, including metastasis, primary neoplasm, direct extension of adjacent neoplasm, diffuse or focal degeneration, or inflammatory conditions, and so on. For these reasons, robust and uniform fat suppression, especially on T2W images, is extremely important for radiologists to achieve accurate diagnoses MR imaging of the spine.
Many techniques exist for obtaining fat-suppressed images. FSE imaging with frequency-selective fat saturation pulses is routinely used in spine MR imaging. However, achieving uniform and reliable fat suppression in the spine with this method is difficult due to main magnetic field (B0) and radiofrequency field (B1) inhomogeneities. This is particularly true in some situations such as off-isocenter imaging, large field of view (FOV) imaging, air-tissue interfaces and the presence of metallic hardware (9). Short-inversion-time inversion-recovery (STIR) imaging provides uniform fat suppression; however, STIR is sensitive to B1 inhomogeneities, requires additional time for inversion pulses and suffers from a low signal-to-noise ratio (SNR) (10). Spectral spatial pulses can be used with FSE for fat suppression. Although this method is insensitive to B1 inhomogeneities, it is relatively sensitive to B0 inhomogeneities (11).
The original two-point Dixon method was first described by Dixon (12) in 1984, and was used for fat and water separation by utilizing differences in chemical shifts, but was sensitive to field inhomogeneities. In order to demodulate field inhomogeneity effects from the acquired source images, a three-point method with symmetrically acquired echoes was developed to measure the inhomogeneities directly, which renders this method insensitive to both B0 and B1 inhomogeneities (13-15). It was recently demonstrated, however, that decomposition of water from fat with symmetrically acquired echoes could not be achieved when the proportions of water and fat within a voxel are approximately equal (16).
Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) is a cutting edge modified three-point method choosing asymmetric echo shifts to create relative water-fat phase shifts of -π/6, π/2, 7π/6 (9, 17). This approach has been demonstrated to provide uniform and reliable fat suppression and has been successfully combined with FSE (9), gradient-recalled acquisition in steady-state (GRASS) imaging (18), spoiled gradient-recalled echo (SPGR) (19), and balanced steady-state free precession (SSFP) techniques (20) to create high-quality fat-suppressed MR images.
Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) imaging has been applied with robust water-fat separation throughout the body, including the head and neck (9, 21), breast (22), heart (22, 23), abdomen (9, 22), pelvis (9), and extremities (9, 18, 20, 24). However, to our knowledge, only a few studies have focused the application of IDEAL in spine MR imaging (6, 9, 25), and no previous MR imaging study of the spine with T2-weighted IDEAL fast recovery fast spin-echo (FRFSE) at 3.0 Tesla (T) has been reported.
The aim of this work was to evaluate T2W IDEAL FRFSE imaging of the spine at 3.0T under different limited situations. In thirty-five patients, we compared T2W IDEAL FRFSE with fat-saturated T2W FRFSE imaging. Quantitative measurements of SNR efficiency and qualitative scoring of diagnostic image quality and fat suppression were performed.
Between October and December 2010, 35 consecutive patients (M:F = 18:17; mean age, 59.2 years; range, 22-84 years) were referred by their physicians for spine evaluation of known metastasis in eight patients, degeneration of disk in 17 patients, compression of the vertebral body in eight patients, and metallic thoracic spine fixation plates with screws in two patients with history of trauma. MR imaging of the cervical (8 cases), thoracic (8 cases), lumbar (19 cases) spine was performed at a 3.0T GE Healthcare Signa HDx MR scanner (GE Medical Systems, Milwaukee, WI) using an 8-channel CTL phased-array target coil. This study was approved by our Institutional Review Board and informed consent was obtained from all patients.
Images were obtained as a part of the routine spine protocol at our institution. Comparison images between T2W IDEAL FRFSE and fat-saturated T2W FRFSE were obtained in the sagittal plane only. GE FRFSE-IDEAL pulse sequence and reconstruction software were used. T2WI IDEAL FRFSE image parameters included repetition time (TR)/echo time (TE) (eff), 2200/85 milliseconds; bandwidth, +62.5 kHz; echo-train length, 14; FOV, 30 cm; slice/gap thickness, 4/1 mm; 11 sagittal images; 320 × 192 matrix; and the number of excitations (NEX), 2. Total imaging time was 3:18 minutes. Fat-saturated T2W FRFSE images were obtained at the same locations with the identical image parameters except for TE (102 milliseconds), and NEX of 4, for a total scanning time of 2:12 minutes. Product-automated shim routines were used for all of the spine imaging.
Slight differences in TE were caused by differences in the time between refocusing pulses resulting from the need to shift echoes slightly for IDEAL (6). The raw IDEAL images were reconstructed on line to acquire water-only, fat-only, and recombined in-phase and out-of-phase images in approximately 1 minute.
Signal measurements were made in vertebral bodies and spinal cord in the mid-sagittal plane or nearest to the mid-sagittal plane for T2W IDEAL FRFSE water-only and fat-saturated T2W FRFSE images. All measurements were made by the same radiologists. The circular region of interest (ROI) for the signal measurements was 80-100 pixels in size. It was identical in size and location for all measurements within an individual, but was tailored slightly in size and eccentricity to account for anatomic differences between individuals. A circular ROI comprised of approximately 300 pixels for noise measurements was placed in a region that is outside the body free of phase ghosting artifact. The same ROIs for both signal and noise were used at the same location to ensure accurate SNR-efficiency comparison between T2W IDEAL FRFSE and fat-saturated T2W FRFSE. ROIs were usually placed in C4, L3, T6 vertebral bodies and corresponding spinal cord respectively, and only in different segments when avoiding regions of inhomogeneous fat saturation and metal artifacts. SNR-efficiencies of the vertebral bodies and spinal cord were computed. SNR was defined as the ratio of the average signal intensity over the standard deviation of the noise. SNR-efficiency was the ratio of SNR to the square-root of total scanning times. SNR efficiencies were calculated to provide a fair measure of SNR that corrects for differences in scanning times between the two sequences.
Subjective scoring of the images by two experienced radiologists (with 11 and 18 years experience in clinical MRI, respectively) was done by consensus on a four-point grading scale for fat suppression and overall diagnostic image quality (0, poor; 1, fair; 2, good; 3, excellent) (24). The reviewers were blinded to the type of acquisition they were grading. Comparisons between images for image quality and fat suppression or fat-water separation were done with the T2W IDEAL FRFSE water-only images and the fat-saturated T2W FRFSE images only.
All statistical analyses was performed using a statistical software (SPSS, version, 13.0; Chicago, IL). For all statistical tests, a p value of less than 0.05 was considered to indicate a significant difference. Comparisons between the two imaging sequences were performed by applying a paired sample Student's t test to the SNR-efficiency. The mean scores of the two radiologists for T2WI IDEAL FRFSE and fat-saturated T2WI FRFSE sequences were compared for image quality and uniformity of fat-water separation or fat suppression using a Wilcoxon's signed rank test.
Successful separation of water and fat was achieved in all studies for T2W IDEAL FRFSE imaging. The IDEAL water-only images showed robust and uniform fat suppression and high SNR-efficiency performance (Fig. 1A). Separate IDEAL fat-only images and recombined IDEAL in-phase images had corrected chemical shift artifacts (Fig. 1B-D).
Signal-to-noise ratio-efficiency of the vertebral body was significantly different between IDEAL (2.83 ± 0.28) and fat-saturated FRSE (1.82 ± 0.28), and SNR-efficiency of spinal cord was also significantly higher for IDEAL FRFSE (8.69 ± 0.63) compared with fat-saturated FRFSE imaging (5.99 ± 0.35) (p < 0.05).
Overall image quality score for T2WI IDEAL FRFSE (2.9) was higher (p < 0.01) than that for fat-saturated T2W FRFSE (2.1) imaging. Fat suppression was rated as fair to good on fat-saturated T2W FRFSE images (1.8), but fat-water separation was excellent (2.9) on the T2W IDEAL FRFSE images. The difference between fat suppression and fat-water separation using IDEAL was statistically significant (p < 0.01).
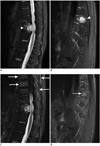
Fat-water separation with IDEAL FRFSE imaging performed particularly well in the presence of metal (Fig. 2) and some challenge regions (Fig. 3), showing fewer artifacts than fat-saturated FRFSE imaging. In some cases, areas of poor fat suppression on fat-saturated FRFSE images were seen mimicking traumatic bone marrow edema (Fig. 4) or metastasis (Fig. 5), but superior fat-water separation in the corresponding water-only images showed that these were artifacts caused by poor fat saturation.
It is well known that T2W sequences with fat suppression are very important for radiologists to discriminate a variety of pathologies in the spine. Unfortunately, we occasionally have situations in which, despite careful shimming, fat signal intensity is inadequately suppressed using conventional methods. Murakami et al. (25) have demonstrated that IDEAL can provide more uniform fat suppression and spinal canal projection with fewer artifacts from magnetic inhomogeneity compared with fat-suppressed FSE using chemical shift selective saturation (CHESS). In our work, T2W IDEAL FRFSE imaging reliably achieved excellent fat suppression in areas susceptible to consistent failure of fat suppression on the fat-saturated T2W FRFSE images-near not only the presence of metallic hardware, but also the air-tissue interfaces, off-isocenter and top margin of FOV. Meanwhile, IDEAL images showed superior image quality and higher SNR-efficiency performance compared with fat-saturated T2W FRFSE images.
In addition to water-only images, IDEAL provides multiple contrasts including fat-only and recombined fat/water in-phase and out-of-phase images from one acquisition. Although our study did not focus on the recombined images, the recombined in-phase IDEAL FRFSE images with chemical shift correction are of high quality and provide anatomic reference similar to conventional T2W images, which may permit elimination of conventional T2W image from the MRI protocols. Thus, a single acquisition with IDEAL imaging has the potential to simplify spine MRI protocols by replacing separate acquisitions that use fat-saturation and chemical shift techniques. Furthermore, because all data emanate from a single acquisition, the resulting diverse image sets are perfectly coregistered. IDEAL imaging possibly reduces "on-the-table" time for patients and improves the cost-effectiveness of MRI. Further study is required to determine whether removing the T2 or T1 weighted images from a spine protocol can result in the loss of important diagnostic information or not. Another benefit of IDEAL imaging is that in-phase and out-of-phase technique may be useful for differentiating benign from neoplastic processes within bone marrow by detecting small amounts of microscopic fat within benign lesions (26). Advantages of the IDEAL in-phase and out-of-phase images need to be fully explored in the future study.
In recent years, many institutions have increasingly operated 3.0T MR imaging systems. One challenge of 3.0T spine imaging is the increased chemical artifacts due to a doubling of the difference in the resonance frequency between fat and water protons from 1.5T to 3.0T. Imaging at 3.0T often requires increases in bandwidth to avoid the increased chemical shift artifacts, which result in low SNR performance. Correction of chemical shift with IDEAL imaging at 3.0T eliminates the need to increase bandwidth and better preserving the SNR benefit of 3.0T imaging. Another challenge of 3.0T is that susceptibility to magnetic field inhomogeneities increases, which give rise to more artifacts and failure of fat suppression in some situations. IDEAL imaging can overcome the effect of increased susceptibility to magnetic field inhomogeneities and provides robust fat suppression and recombined images. The full benefits of these advantages of IDEAL at 3.0T will require additional evaluation on more subjects in future studies.
In general, SNR and subjective quality comparisons are best done for 2 sequences with nearly the same imaging parameters and the same amount of time. However, IDEAL requires as much acquisition time as do 3 numbers of excitations because it needs 3 echo times, hence taking about triple the time than the fat-saturated sequence for 1 scan under identical imaging parameters. In order to achieve impartial and accurate comparisons, we used a NEX of 4 in a fat-saturated sequence to double the scanning time approximately to the time of IDEAL, which led to an increase of SNR in a fat-saturated sequence. If we changed NEX to 2 and imaging time to 1:06 for the fat-saturated FRFSE sequence, the SNR would be lower, image quality would be worse and SNR-efficiency would not change according to the effect of imaging parameters on SNR. However, in routine practice, we can expect the advantage of fast imaging for the fat-saturated FRFSE sequence, and diagnosis may be easily made on fat-saturated T2 FRFSE images with a NEX of 2.
The main disadvantage of IDEAL is an increase in the scanning time because of the requirement of three acquisitions to separate water from fat. There have been several methods employed to reduce scanning time including parallel imaging, reduced sampling strategies, and the use of half k-space acquisitions and homodyne reconstruction (6, 9, 24). However, the scanning times at 1.5T were still 5-6 minutes in some literatures (6, 24). The FRFSE sequence used in our study is a modified FSE sequence. In FRFSE, transverse magnetization is still present at the end of the echo train, and then a -90° pulse refocuses protons back into the longitudinal axis rather than allowing them to undergo T1W recovery. This reduces the transverse magnetization in the longitudinal axis, resulting in an acceleration of the relaxation of longitudinal magnetization. FRFSE sequences have shorter TR with comparable contrast and a reduced imaging time. This reduction in TR improves SNR efficiency and also offsets the increased minimum scanning time required for the IDEAL technique, and in the meantime, benefited from the comprehensive technical progresses using a GE HDx MR scanner. Moreover, IDEAL combined with FRFSE can be effective in alleviating the long scanning time to approximately 3 minutes, even with 2 NEX and SNR-efficiency improvement. Although the imaging time of the IDEAL sequence is still longer than that of fat-saturated FRFSE imaging due to IDEAL's inherent characteristic, IDEAL imaging has the potential to simplify spine MRI protocols by replacing some acquisitions, and the overall scanning times of spine MRI may be reduced along with excellent fat suppression and high SNR-efficiency. Times are, so to speak, no longer clinically limiting for IDEAL FRFSE imaging.
For the fixed bandwidths received in our study, T2 decay will increase at the later echoes and may worsen blurring for imaging because of the increased time required to acquire a train of echoes. The increased T2 weighting with echoes acquired at the end of the echo train may actually increase the edge enhancement that occurs with T2W FSE imaging. However, different from FSE, FRFSE uses -90° refocusing pulses that elongate echo trains to generate better contrast between long and short T2 species with little blurring and low radiofrequency power. The overall effect of the longer echo train with IDEAL depends on the particular k-space ordering and effective TE chosen. The longer echo train has no appreciable effect on image quality on either T1W or T2W IDEAL-FSE imaging (6), and also on FRFSE imaging.
In conclusion, T2W IDEAL FRFSE imaging is a promising technique for spine MR imaging at 3.0T because it provides consistent, robust and uniform fat suppression compared with conventional fat-saturated FRFSE imaging, while possessing high SNR-efficiency and image quality. Additional studies should be done to assess the performance of IDEAL in discrimination of different pathologies and fat quantification.
Figures and Tables
Fig. 1
41-year-old woman with lower back pain.
A. Sagittal T2-weighted iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) fast recovery fast spin-echo (FRFSE) water-only image. B. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) fat-only image. C. Recombined iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) in-phase image. D. Recombined iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) out-of-phase image. Separate water (A) and fat (B) images demonstrate uniform separation of water and fat. Correction of chemical shift artifacts is shown in recombined in-phase image (C).
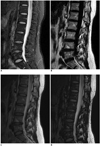
Fig. 2
26-year-old man with history of surgery after traffic accident.
A. Sagittal T2-weighted iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) fast recovery fast spin-echo (FRFSE) water-only image. B. Sagittal fat-saturated T2-weighted fast recovery fast spin-echo (FRFSE) image. Failed fat-saturation and susceptibility artifacts due to B0 field inhomogeneities generated from metallic hardware in several thoracic vertebral bodies that obscured large portion of spinal cord and vertebral body (arrows). Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) image demonstrated very uniform fat separation despite presence of metallic hardware (A).
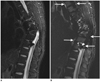
Fig. 3
56-year-old man with neck pain.
A. Sagittal T2-weighted iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) fast recovery fast spin-echo (FRFSE) water-only image. B. Sagittal fat-saturated T2-weighted fast recovery fast spin-echo (FRFSE) image. Note failure of fat suppression in areas with air-tissue interfaces, and unfavorable geometry on fat-saturated fast recovery fast spin-echo (FRFSE) image (arrows), but uniformity of water-fat separation on water-only image was demonstrated (A).
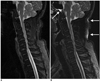
Fig. 4
76-year-old woman with history of trauma.
A. Sagittal T2-weighted iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) fast recovery fast spin-echo (FRFSE) water-only image. B. Sagittal fat-saturated T2-weighted fast recovery fast spin-echo (FRFSE) image. Compression of T11 vertebral body is seen (arrows). Poor fat suppression on fat-saturated fast recovery fast spin-echo (FRFSE) image in areas near top margin of field of view is seen mimicking T5 vertebral body marrow edema (dashed arrow), but superior fat-water separation and signal-to-noise ratio performance in corresponding iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) water-only image show that this is artifact caused by poor fat saturation (A).
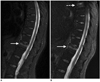
Fig. 5
56-year-old man with 12-year history of lung adenocarcinoma.
A, B. Sagittal T2-weighted iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) fast recovery fast spin-echo (FRFSE) water-only images. C, D. Corresponding fat-saturated T2-weighted fast recovery fast spin-echo (FRFSE) images. This patient has multiple metastases in spine. Fat-saturated T2-weighted fast recovery fast spin-echo (FRFSE) images demonstrate unreliable fat suppression in superior regions of images due to off-isocenter B0 inhomogeneities (arrows), which obscured lesion in left spinal facet joint at T9/T10 (dashed arrow). All lesions are distinctly visualized on iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) water-only images because of uniform and reliable fat suppression (arrowheads).
References
1. Vertinsky AT, Krasnokutsky MV, Augustin M, Bammer R. Cutting-edge imaging of the spine. Neuroimaging Clin N Am. 2007. 17:117–136.
2. Alyas F, Saifuddin A, Connell D. MR imaging evaluation of the bone marrow and marrow infiltrative disorders of the lumbar spine. Magn Reson Imaging Clin N Am. 2007. 15:199–219.
3. Nobauer I, Uffmann M. Differential diagnosis of focal and diffuse neoplastic diseases of bone marrow in MRI. Eur J Radiol. 2005. 55:2–32.
4. Vanel D, Dromain C, Tardivon A. MRI of bone marrow disorders. Eur Radiol. 2000. 10:224–229.
5. D'Aprile P, Tarantino A, Jinkins JR, Brindicci D. The value of fat saturation sequences and contrast medium administration in MRI of degenerative disease of the posterior/perispinal elements of the lumbosacral spine. Eur Radiol. 2007. 17:523–531.
6. Reeder SB, Yu H, Johnson JW, Shimakawa A, Brittain JH, Pelc NJ, et al. T1- and T2-weighted fast spin-echo imaging of the brachial plexus and cervical spine with IDEAL water-fat separation. J Magn Reson Imaging. 2006. 24:825–832.
7. Zha Y, Li M, Yang J. Dynamic contrast enhanced magnetic resonance imaging of diffuse spinal bone marrow infiltration in patients with hematological malignancies. Korean J Radiol. 2010. 11:187–194.
8. Oner AY, Akpek S, Tali T, Ucar M. Giant vertebral notochordal rest: magnetic resonance and diffusion weighted imaging findings. Korean J Radiol. 2009. 10:303–306.
9. Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005. 54:636–644.
10. Bydder GM, Pennock JM, Steiner RE, Khenia S, Payne JA, Young IR. The short TI inversion recovery sequence--an approach to MR imaging of the abdomen. Magn Reson Imaging. 1985. 3:251–254.
11. Block W, Pauly J, Kerr A, Nishimura D. Consistent fat suppression with compensated spectral-spatial pulses. Magn Reson Med. 1997. 38:198–206.
12. Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984. 153:189–194.
13. Glover GH. Multipoint Dixon technique for water and fat proton and susceptibility imaging. J Magn Reson Imaging. 1991. 1:521–530.
14. Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magn Reson Med. 1991. 18:371–383.
15. Hardy PA, Hinks RS, Tkach JA. Separation of fat and water in fast spin-echo MR imaging with the three-point Dixon technique. J Magn Reson Imaging. 1995. 5:181–185.
16. Pineda AR, Reeder SB, Wen Z, Pelc NJ. Cramer-Rao bounds for three-point decomposition of water and fat. Magn Reson Med. 2005. 54:625–635.
17. Reeder SB, Wen Z, Yu H, Pineda AR, Gold GE, Markl M, et al. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn Reson Med. 2004. 51:35–45.
18. Kijowski R, Blankenbaker DG, Woods MA, Shinki K, De Smet AA, Reeder SB. 3.0-T evaluation of knee cartilage by using three-dimensional IDEAL GRASS imaging: comparison with fast spin-echo imaging. Radiology. 2010. 255:117–127.
19. Reeder SB, McKenzie CA, Pineda AR, Yu H, Shimakawa A, Brau AC, et al. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 2007. 25:644–652.
20. Gold GE, Reeder SB, Yu H, Kornaat P, Shimakawa AS, Johnson JW, et al. Articular cartilage of the knee: rapid three-dimensional MR imaging at 3.0 T with IDEAL balanced steady-state free precession--initial experience. Radiology. 2006. 240:546–551.
21. Barger AV, DeLone DR, Bernstein MA, Welker KM. Fat signal suppression in head and neck imaging using fast spin-echo-IDEAL technique. AJNR Am J Neuroradiol. 2006. 27:1292–1294.
22. Yu H, Reeder SB, McKenzie CA, Brau AC, Shimakawa A, Brittain JH, et al. Single acquisition water-fat separation: feasibility study for dynamic imaging. Magn Reson Med. 2006. 55:413–422.
23. Reeder SB, Markl M, Yu H, Hellinger JC, Herfkens RJ, Pelc NJ. Cardiac CINE imaging with IDEAL water-fat separation and steady-state free precession. J Magn Reson Imaging. 2005. 22:44–52.
24. Fuller S, Reeder S, Shimakawa A, Yu H, Johnson J, Beaulieu C, et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) fast spine-cho imaging of the ankle: initial clinical experience. AJR Am J Roentgenol. 2006. 187:1442–1447.
25. Murakami M, Mori H, Kunimatsu A, Abe O, Chikuda H, Ono T, et al. Postsurgical spinal magnetic resonance imaging with iterative decomposition of water and fat with echo asymmetry and least-squares estimation. J Comput Assist Tomogr. 2011. 35:16–20.
26. Zajick DC Jr, Morrison WB, Schweitzer ME, Parellada JA, Carrino JA. Benign and malignant processes: normal values and differentiation with chemical shift MR imaging in vertebral marrow. Radiology. 2005. 237:590–596.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download