Abstract
An epithelioid hemangioma involving three contiguous bones in continuity has, to the best of our knowledge, not been reported in the literature. A case of a 48-year-old man presented with radiating pain to the lower thoracic region for two years. A radiograph and CT scan revealed both permeative osteolytic and multiple trabeculated lesions involving the left posterior part of the 10th rib as well as the 9th and 10th vertebral bodies in continuity and was misled as a malignant or infectious lesion. The histopathology and immuno-histochemistry of the lesion confirmed the diagnosis of an epithelioid hemangioma. The lesion was still stable as of three years after surgery.
An epithelioid hemangioma involving three contiguous bones in continuity as seen in our case is rare and, to our best knowledge, has not been addressed in the literature. The findings can lead to be misinterpreted as a malignant tumor or chronic infectious disease by conventional X-rays, CT scans and even magnetic resonance imaging (MRI). Consequently, a percutaneous biopsy is mandatory to make a correct diagnosis and determine the appropriate course of treatment.
A 48-year-old man presented with radiating pain to lower thoracic region for two years. A physical examination was unremarkable and he had no significant past medical history. Plain radiographs of the thoracic spine revealed a lobulated geographic osteolytic lesion with a partially sclerotic border involving the left side of the T10 vertebral body, which continued up to involve the left side of the T9 vertebral body. The T9-10 disk space was narrow, suggesting disease involvement. Also observed was a permeative osteolytic lesion involving the posterior part of the left 10th rib at the costo-vertebral junction, associated with a soft tissue mass (Fig. 1A, B). The radiographic findings of the permeative osteolytic lesion and involvement of more than two bones, raised the possibility of malignancy, particularly the metastatic deposit. A CT scan revealed a well-defined osteolytic lesion with cortical expansion involving the left side of the T10, which continued up through the narrow T9-10 disk, and also involved the left side of the T9 vertebral body. The lesion caused spinal cord compression and involved almost the entire T10 vertebral body, mainly the posterior portion, along with the posterior part of the left 10th rib adjacent to the costo-vertebral junction. The lesion contained multiple internal trabeculations as well as multiple scattered tiny calcifications. The soft tissue component also formed an extrapleural mass posterior to the thoracic aorta (Fig. 1C-G). In this clinical setting, either infectious process, especially tuberculosis which is rather common in Thailand or metastatic deposits was suspected. As a result, tests were performed to identify primary cancer. An ultrasound of the abdomen showed a normal sized liver without evidence of a mass or intrahepatic ductal dilatation. No remarkable finding was revealed after examining the pancreas, spleen, and both kidneys. The patient's PSA level was within normal limits (1.31 ng/L) and a transurethral prostatectomy specimen revealed benign prostatic hyperplasia. A peripheral blood examination revealed the following: WBC 8.2-15 K/UL (4.8-10.8), RBC 2.9-5.2 M/µl (4.2-6.1), neutrophils 30-86% (40-74), eosinophils 23% (0-7), basophils 2% (0-1.5). Excisional biopsies performed on the T9 and T10 vertebrae revealed multiple pieces of bone tissue and grey brown soft tissue (measuring 1.5×0.9×0.4 cm in T9 and 10×5×4.5 cm in T10 in aggregate). Microscopically, the lesion showed a circumscribed lobular lesion made up of a different sized gland-like or tubular structure lined with plump cuboidal cells and occasional hop-nail projections into luminal space. In addition, tall and enlarged endothelial cells imparting so-called 'tombstone-like features' were seen projected into the lumen (Fig. 1H, I). The lesion also illustrated the transition from solid cords to tubules forming lumens containing blood recapitulating vessel-formations. The immunostains of the lining cells showed strong immuno-reactivity for cytokeratin (AE1/AE3) and vascular markers CD31, CD 34, and Factor VIII-related antigen (Fig. 1J). As a result the epithelioid hemangioma should be considered as part of the differential diagnoses. A laminectomy and spinal fusion was performed and found that the disease was still stable after three years.
According to the 2000 WHO classification, epithelioid hemangioma is recognized as a benign entity and separated from hemangioendothelioma which was considered a borderline or malignant lesion (1). The literature indicates that an epithelioid hemangioma of bone is rare and about one-fifth of cases showed multiple lesions, most of which were not located in contiguous bone (2). Our case appears to be the first case showing continuous lesions involving three contiguous bones and may make it difficult to exclude malignancy and chronic infectious disease, especially tuberculosis which is quite common and can cause severe destruction of contiguous vertebral bone (3). As described by O'Connell et al. (4), five out of 10 cases of epithelioid hemangioma showed complete osteolytic lesions with well-defined sclerotic borders; the remaining five cases showed a mixed lytic and sclerotic appearance with partial cortical destruction and thick periosteal reactive bone formation. Ben Romdhane et al. (5) and Rosai et al. (6) also reported well-defined lesions with sclerotic margins, while Ling et al. (7) described a diffuse sclerotic lesion. Our case is distinct from others because 1) the lesion involves three bones in contiguity: the T10 and T9 vertebral bodies including the left 10th rib, 2) two patterns of bone destruction were detected from a plain radiograph: the geographic (in the vertebral bodies) and the permeative osteolytic lesion (of the 10th rib), and 3) our case showed cortical bone destruction accompanied by a soft tissue component, but without a definite periosteal reaction. The differential diagnosis in the plain radiograph included a giant cell tumor, an aneurysmal bone cyst, a brown tumor, an infectious spondylitis, and metastatic deposits. These entities can appear as an osteolytic expanding lesion. The permeative osteolytic lesion that involved the left 10th rib included the possibility of an infection and metastasis which was more concerning. However, in the plain radiographs, we could not demonstrate that the lesions in the spine and the rib were continuous. It was the subsequent CT scan that showed the continuity and narrowed the differential diagnosis in our case to only include a chronic infectious process and metastatic deposits. For the infectious process, notably tuberculosis typically shows subligamentous extension. Although this imaging sign was absent in our case, spinal tuberculosis cannot be entirely excluded due to the fact that 85% of the patients showed this sign, while the other 15% did not (8). We included metastasis in our differential diagnosis because of the permeative osteolytic lesion at the posterior portion of the left 10th rib in the plain radiograph. The rib lesion continued with the spinal lesion, and as a result, considered them as the same entity and proposed that metastasis might be included in the differential list, even though it is unlikely for metastasis to involve the intervertebral disk.
Regarding the findings of epithelioid hemangioma by CT scan, Ling et al. (7) reported that a contrast-enhanced CT scan revealed an expanding lytic lesion involving the right anterolateral aspect of the T7 vertebral body. The lesion had a thin ossification rim at its extraosseous periphery, and also contained scattered calcifications and residual trabeculae. The remainder of the vertebral body exhibited generalized diffuse reactive sclerosis which was most prominent at the interface with the lesion. In our case, the findings differ in that the lesion did not have thin rim of ossification at its extraosseous periphery (Fig. 1C-G). However, our lesion contained scattered tiny calcifications and internal trabeculations consistent with the findings in Ling et al. (7). The remainder of the vertebral body in our case showed normal vertebral density, which differed from the report by Ling et al. (7).
From an imaging aspect, it was unlikely for our case to be a paravertebral soft tissue lesion involving two adjacent vertebrae and a rib. We found in the CT scan, in particular (Fig. 1E-G), that the bones showed expansion at the left posterior aspect of the T10 vertebral body and the posterior portion of the left 10th rib. These findings indicated that the lesions were originally intraosseous that expanded the bones, subsequently broke the cortex and formed the extraosseous component.
The patient in this case study was followed up for about three years and found that the lesion was still stable. Similar to other authorities, we believe that epithelioid hemangiomas can elicit bone resorption and cause local destruction in spite of being a benign lesion (6). Since the lesion in our case appeared rather aggressive, it is noteworthy to be aware of a falsely malignant diagnosis to avoid unnecessary aggressive treatments. Our case showed peripheral blood eosinophilia and it was also found in about half of the reported cases (2). This may provide a clue to clinicians to include this entity in the differential diagnosis when they encounter patients with coexisting blood eosinophilia and bone lesions. Histologically, the lesion can mimic adenocarcinomas and other malignant vascular tumors including angiosarcomas and hemangioendotheliomas. Both adenocarcinomas and epithelioid hemangiomas can yield positive immunoreactivity for cytokeratins, which are usual markers for epithelial tumors. However, in our case, in addition to showing positive for epithelial marker (AE1/AE3), they also exhibited a positive result for vascular markers (CD31 and CD34), which should not be positive in the case of an adenocarcinoma. The misleading diagnosis of this lesion as a case of adenocarcinoma is possible since the plump endothelial cells look quite similar to epithelial cells of an adenocarcinoma and this diagnostic pitfall might occur if only immuno-stainings for epithelial markers were performed. The rather well circumscribed lesion including the reactive phenomenon of markedly eosinophilic infiltration and lymphoid follicle formations in the surrounding tissue may be an additional feature to stimulate the awareness of this entity and to further prove it with aforementioned immunostains.
Some histologic viewpoints were considered in differentiating between an epithelioid hemangioma and a hemangioendothelioma (9-12): 1) epithelioid hemangiomas tends to show circumscribed lesions, while hemangioendotheliomas often show infiltrative borders, 2) the cells in epithelioid hemangiomas tend to show a lumen formation imparting tubular structure whereas hemangioendothelioma often arrange in clusters, nests, and cord-like structure, 3) epithelioid hemangiomas often show an inflammatory reaction which is an especially prominent eosinophilic infiltration, while hemangioendotheliomas lack this feature, 4) hemangioendotheliomas often possess myxoid or hyalinized stroma, and 5) hemangioendotheliomas often lack plump endothelial cells protruding into the lumen imparting a 'tombstone-like' feature. Our case possesses all aforementioned features that support the diagnosis of an epithelioid hemangioma. Angiosarcomas generally show marked atypia, frequent mitosis, infiltrative borders; and necrosis. Our present case lacks all of these features and therefore, the diagnosis of angiosarcoma is very unlikely (4, 9, 12).
In conclusion, we report a case of an epithelioid hemangioma involving three contiguous bones and mimicked malignancy or chronic infectious disease in the plain film and CT imagings. A biopsy is therefore highly recommended when encountering such a lesion. The familiarity and awareness of such an entity also helps pathologists to make a correct diagnosis.
Figures and Tables
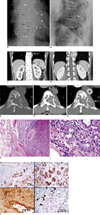
Fig. 1
Epithelioid hemangioma in 48-year-old man.
Plain radiographs. Anteroposterior (AP) view (A) reveals geographic osteolytic lesion with partially sclerotic border involving left side of T10 vertebral body. Another similar osteolytic lesion is also observed on left side of T9 vertebral body (black arrows). In addition, permeative osteolytic lesion involving posterior part of left 10th rib at costo-vertebral junction (thin white arrows), associated with soft tissue mass (thick white arrows) was observed. No periosteal reaction is observed. Lateral view (B) reveals lesion involving posterior portion of T10 vertebral body (black arrows) with extension to posterior element (thin white arrows). Osteolytic lesion is also seen involving posterior portion of T9 vertebral body (thick white arrow).
C-G. Coronal CT scan (C, D) after iodinated contrast medium injection reveals that lesion involves two contiguous vertebral bodies, T10 and T9 (arrow in C), which causes spinal cord compression (arrow in D). Axial CT scan at T10 vertebral body viewing in bone window (E) and soft tissue window before (F) and after (G) contrast medium injection reveals multiple internal trabeculations, multiple scattered tiny calcifications, and mild enhancement of lesion. Left-side cortex of T10 vertebral body and ventral cortex of 10th rib were destroyed with soft tissue component forming as extrapleural mass (arrow in G) behind thoracic aorta (star).
H. Photomicrograph of lesion under low power (×40) reveals well-circumscribed lobular lesion made up of crowded small tubular or gland-like structure surrounded centrally by large vessel with thick wall. Periphery of lesion is surrounded with fibrofatty tissue.
I. Under ×400 magnification, lining cells of gland-like structure show irregular vesicular or optically clear nuclei with prominent basophilic nucleoli and occasional vacuolated cytoplasm. Tall and enlarged endothelial cell mimicking 'tomb-stone like structure' projected into lumen, is observed (arrow).
J. Immunostains using antibodies reacting to epithelial marker (CK) and vascular markers (CD31, CD34 and Factor VIII RA), show that lining cells are strongly marked by all of these antibodies.
References
1. Fetsch J. Fletcher C, Unni K, Mertens F, editors. Epitheloid haemangioma. WHO classification of tumors: pathology and genetics of tumors of soft tissue and bones. 2000. Lyon: IARC Press;159–160.
2. Sung MS, Kim YS, Resnick D. Epithelioid hemangioma of bone. Skeletal Radiol. 2000. 29:530–534.
3. Shanley DJ. Tuberculosis of the spine: imaging features. AJR Am J Roentgenol. 1995. 164:659–664.
4. O'Connell JX, Kattapuram SV, Mankin HJ, Bhan AK, Rosenberg AE. Epithelioid hemangioma of bone. A tumor often mistaken for low-grade angiosarcoma or malignant hemangioendothelioma. Am J Surg Pathol. 1993. 17:610–617.
5. Ben Romdhane K, Khattech R, Ben Othman M. Epithelioid hemangioma of bone. Am J Surg Pathol. 1994. 18:1270–1271.
6. Rosai J, Gold J, Landy R. The histiocytoid hemangiomas. A unifying concept embracing several previously described entities of skin, soft tissue, large vessels, bone, and heart. Hum Pathol. 1979. 10:707–730.
7. Ling S, Rafii M, Klein M. Epithelioid hemangioma of bone. Skeletal Radiol. 2001. 30:226–229.
8. Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. AJR Am J Roentgenol. 2004. 182:1405–1410.
9. Weiss SW, Goldblum JR. Weiss SW, Goldblum JR, editors. Hemangioendothelioma: vascular tumors of intermediate malignancy. Enzinger and Weiss's soft tissue tumors text book. 2008. Philadelphia: Mosby Elsevier;681–686.
10. Weiss SW, Goldblum JR. Weiss SW, Goldblum JR, editors. Epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia, histiocytoid hemangioma). Enzinger and Weiss's soft tissue tumors text book. 2008. Philadelphia: Mosby Elsevier;644–649.
11. Montgomery E. Silverberg SG, DeLellis RA, Frable WJ, editors. Hemangioendothelioma. Silverberg's principles and practice of surgical pathology and cytopathology. 2006. Philadelphia: Churchill Livingstone Elsevier;365–368.
12. Nielsen GP, Srivastava A, Kattapuram S, Deshpande V, O'Connell JX, Mangham CD, et al. Epithelioid hemangioma of bone revisited: a study of 50 cases. Am J Surg Pathol. 2009. 33:270–277.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download