1. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011; 469:2706–2715.
2. Lyras DN, Kazakos K, Verettas D, Polychronidis A, Tryfonidis M, Botaitis S, Agrogiannis G, Simopoulos C, Kokka A, Patsouris E. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009; 30:1101–1106.
3. Kark LR, Karp JM, Davies JE. Platelet releasate increases the proliferation and migration of bone marrow-derived cells cultured under osteogenic conditions. Clin Oral Implants Res. 2006; 17:321–327.
4. Debus ES, Schmidt K, Geiger D, Dietz UA, Thiede A. Growth factors for preventing amputation in delayed wound healing. Kongressbd Dtsch Ges Chir Kongr. 2001; 118:829–833.
5. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010; 38:255–262.
6. Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autologous platelet rich plasma for arthroscopic rotator cuff repair. A pilot study. Disabil Rehabil. 2008; 30:1584–1589.
7. Norman J, Tsau YK, Bacay A, Fine LG. Epidermal growth factor accelerates functional recovery from ischaemic acute tubular necrosis in the rat: role of the epidermal growth factor receptor. Clin Sci (Lond). 1990; 78:445–450.
8. Matsumoto K, Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001; 59:2023–2038.
9. Bledsoe G, Crickman S, Mao J, Xia CF, Murakami H, Chao L, Chao J. Kallikrein/kinin protects against gentamicin-induced nephrotoxicity by inhibition of inflammation and apoptosis. Nephrol Dial Transplant. 2006; 21:624–633.
10. Abdelsameea AA, Mohamed AM, Amer MG, Attia SM. Cilostazol attenuates gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2016; 68:247–253.
11. Franco D, Franco T, Schettino AM, Filho JM, Vendramin FS. Protocol for obtaining platelet-rich plasma (PRP), platelet-poor plasma (PPP), and thrombin for autologous use. Aesthetic Plast Surg. 2012; 36:1254–1259.
12. Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970; 26:57–60.
13. Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999; 10:1100–1123.
14. Gamble M. The hematoxylins and eosin. In : Bancroft JD, Gamble M, editors. Theory and Practice of Histological Techniques. 6th ed. Philadelphia, PA: Churchill livingstone/Elsevier;2008. p. 121–126.
15. Weibel ER. Stereological Methods. London: Academic Press;1979.
16. Maldonado PD, Barrera D, Rivero I, Mata R, Medina-Campos ON, Hernández-Pando R, Pedraza-Chaverrí J. Antioxidant S-allylcysteine prevents gentamicin-induced oxidative stress and renal damage. Free Radic Biol Med. 2003; 35:317–324.
17. Choi KH, Kim TI, Chong DL, Lee HY, Han DS. Gentamicin induced apoptosis of renal tubular epithelial (LLC-PK1) cells. Korean J Intern Med. 2000; 15:218–223.
18. Quiros Y, Vicente-Vicente L, Morales AI, López-Novoa JM, López-Hernández FJ. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol Sci. 2011; 119:245–256.
19. Abdeen A, Sonoda H, El-Shawarby R, Takahashi S, Ikeda M. Urinary excretion pattern of exosomal aquaporin-2 in rats that received gentamicin. Am J Physiol Renal Physiol. 2014; 307:F1227–37.
20. Böttinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002; 13:2600–2610.
21. Kim J, Shon E, Kim CS, Kim JS. Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp Diabetes Res. 2012; 2012:210821.
22. Rodriguez-Barbero A, L’Azou B, Cambar J, López-Novoa JM. Potential use of isolated glomeruli and cultured mesangial cells as in vitro models to assess nephrotoxicity. Cell Biol Toxicol. 2000; 16:145–153.
23. Hom DB, Linzie BM, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007; 9:174–183.
24. Lin JJ, Cybulsky AV, Goodyer PR, Fine RN, Kaskel FJ. Insulin-like growth factor-1 enhances epidermal growth factor receptor activation and renal tubular cell regeneration in postischemic acute renal failure. J Lab Clin Med. 1995; 125:724–733.
25. Kempczinski RF, Caulfield JB. A light and electron microscopic study of renal tubular regeneration. Nephron. 1968; 5:249–264.
26. Yang J, Dai C, Liu Y. Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol. 2003; 163:621–632.
27. Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol. 2002; 13:96–107.
28. Brown R, Ollerstam A, Johansson B, Skøtt O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001; 281:R1362–7.

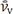 )
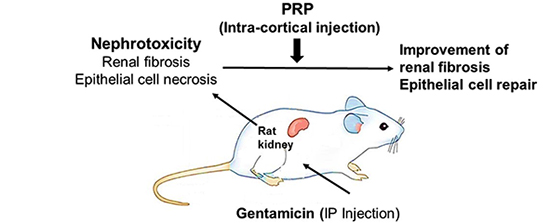
)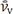 ), from IUR sections. The length of a line, l0, passing through the sampling point that lies within the glomerulus or renal corpuscle to the borders of it (i.e. parietal and visceral layer of Bowman’s capsule) was measured, and the following formula was used for estimation of volume-weighted mean glomerulus and renal corpuscle volume (
), from IUR sections. The length of a line, l0, passing through the sampling point that lies within the glomerulus or renal corpuscle to the borders of it (i.e. parietal and visceral layer of Bowman’s capsule) was measured, and the following formula was used for estimation of volume-weighted mean glomerulus and renal corpuscle volume (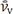 ) (13):
) (13):
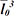 is the average of the cubed linear intercept length across the glomerulus or renal corpuscle through the sampling point.
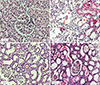
is the average of the cubed linear intercept length across the glomerulus or renal corpuscle through the sampling point. , of proximal and distal convoluted tubule cells was estimated using the optical disector principle. An unbiased counting frame was used to estimate the numerical density of the nuclei. The nuclei of tubule cells located within the frame not touching the exclusion lines and in the defined 18 µm of depth were counted in each field. Nuclei were sampled in 10–14 fields. Numerical density of convoluted tubules was estimated as (13):
, of proximal and distal convoluted tubule cells was estimated using the optical disector principle. An unbiased counting frame was used to estimate the numerical density of the nuclei. The nuclei of tubule cells located within the frame not touching the exclusion lines and in the defined 18 µm of depth were counted in each field. Nuclei were sampled in 10–14 fields. Numerical density of convoluted tubules was estimated as (13):

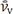 )
)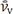 ) diminished (28%) in GM-treated group compared with control group (P = 0.01). There was a significant decrease (26%) in volume-weighted mean renal glomerulus volume in PRP-treated group as compared with GM+NS group (P = 0.03) (Fig. 4D).
) diminished (28%) in GM-treated group compared with control group (P = 0.01). There was a significant decrease (26%) in volume-weighted mean renal glomerulus volume in PRP-treated group as compared with GM+NS group (P = 0.03) (Fig. 4D).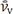 ) decreased (24%) in GM-treated group compared with control group (P = 0.01). There was a significant decrease (30%) in volume-weighted mean renal corpuscle volume in PRP-treated group as compared with GM+NS group (P = 0.04) (Fig. 4E).
) decreased (24%) in GM-treated group compared with control group (P = 0.01). There was a significant decrease (30%) in volume-weighted mean renal corpuscle volume in PRP-treated group as compared with GM+NS group (P = 0.04) (Fig. 4E).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



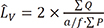
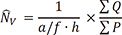
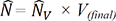
 XML Download
XML Download