Abstract
Glutaric aciduria type I (GA I) is an autosomal recessive disorder caused by a deficiency of glutaryl-CoA dehydrogenase. Although over 400 patients confirmed as GA I have been reported, reports from the Asian population had contributed to the minor proportion. We recently diagnosed two cases of GA I confirmed with mutational analysis. Here, we present their rather atypical clinical presentations with genetic characteristics for the first time in Korea. Profound developmental delay from birth, association of hearing loss, and neurological improvement after surgical intervention, were considered to be different clinical features from most reported cases. One patient was a compound heterozygote for p.Ser139Leu and p.Asp220Tyr, and the other for p.Ser139Leu and Glu160X. The mutations of the two alleles (p.Asp220Tyr and p.Glu160X) were novel and reports of p.Ser139Leu were rare both in Western and other Asian populations. These might suggest different genetic spectrum of Korean GA I patients.
Glutaric aciduria or acidemia type I (OMIM # 231670, GA I) is an inborn error of metabolism caused by a deficiency of glutaryl-CoA dehydrogenase (GCDH) encoded by the GCDH gene. GCDH catalyzes the conversion of glutaryl-CoA (GA) to crotonyl-CoA in the metabolic pathway for lysine, hydroxylysine, and tryptophan (1). An accumulation of GA and 3-OH-GA is established as the biochemical hallmark of GA I, resulting in acute basal ganglia injury, movement disorders, and further neuropsychologic deterioration (2). Since the first description of the two index cases in 1975 (3), over 400 patients and 150 disease causing mutations have been reported (4). The estimated worldwide frequency of GA I is one in 100,000 newborns (5). However, this estimate was based on the tandem mass spectrometry newborn screening results conducted in several countries including the USA, Australia, and Germany. Since GA I is not included in the routine newborn screening program in Korea, exact frequency of this disorder could not be estimated. Only two cases had been reported in the Korean literature diagnosed on the basis of clinical features and results of urine organic acid analysis. Here, we report two Korean patients with GA I, proven by mutational analysis, which revealed two novel mutations, for the first time in Korea.
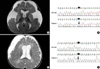
A 10-month-old female infant was referred to our hospital for developmental delay and large head size. She was born to unrelated healthy parents after a 41-week pregnancy. Although she could smile responsively and feed well, complete head control had not been achieved. She had never suffered from metabolic decompensation episodes, such as mental deterioration or seizures associated with infection or fever. Brain magnetic resonance imaging (MRI) revealed large amounts of bilateral subdural fluid collection, cerebral atrophy, and high signal intensity in both basal ganglia (Fig. 1A). After surgical drainage of subdural fluids, recurrent subdural bleeding, systemic infection, decreased mentality, and seizures occurred. These symptoms were not easily controlled in the ensuing postoperative period. Under the suspicion of metabolic encephalopathies, urine organic acid analysis was conducted, which revealed high levels of GA (7,360.9 mM/M Cr, ref: <5.3) and 3-OH-GA (67.6 mM/M Cr ref: <4.2). We analyzed the GCDH gene and identified compound heterozygote mutations of p.Ser139Leu and p.Asp220Tyr (Fig. 1B). Her mother was a heterozygote carrier for p.Ser139Leu mutation and father was a heterozygote carrier for p.Asp220Tyr. Elder brother who was phenotypically normal did not harbor any of the two mutations.
After specific treatment for GA I, including a special protein restriction formula (Glutarex-1, Abbott) with supplementation of L-carnitine (100 mg/kg/day) and riboflavin (100 mg/day), the patient recovered from postoperative complications and her general condition improved. However, her swallowing remained impaired, requiring tube feeding, and her development remained delayed.
A 3-yr-old male, born from unrelated healthy parents after a 39-week pregnancy, was referred to our hospital for developmental delay. Large head size and hearing impairment were detected in the neonatal care unit soon after birth. His motor development was not delayed until six months of age when he could support weight on his forearms and roll over. Subsequently, he lost his motor skills gradually over 1 month without any intervening episodes such as infection or seizures. A brain MRI revealed asymmetric subdural fluid collection, suggesting hemorrhage with a mild mass effect (Fig. 1C). After surgical drainage, his motor skills improved to be able to creep at the age of 13 months. Metabolic screening tests, performed before a cochlear implantation procedure conducted at the age of 29 months, detected highly elevated GA (73.75 mM/M Cr, ref: <5.3) and 3-OH-GA (12.1 mM/M Cr, ref: <4.2) levels from urine organic acid analysis. We analyzed the GCDH gene and found compound heterozygote mutations of p.Glu160X (Fig. 1D) and p.Ser139Leu. The patient was treated with a special formula (Glutarex-1, Abbott), L-carnitine (100 mg/kg/day) and riboflavin (100 mg/day). He can now walk with assistance and did not show any features of movement disorder until his current age of three years.
Genomic DNA was extracted from peripheral blood leukocytes using a Puregene DNA isolation kit (Gentra Systems, Inc., Minneapolis, MN, USA). PCR was performed to amplify the entire coding and flanking regions of all exons of the GCDH gene. The primers were designed using Primer 3 (http://frodo.wi.mit.edu/) and the Refseq of GCDH (NC_000019.8, NM_000159.2). Amplified products were sequenced bidirectionally on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA), then analyzed using the Seqscape v2.5 software. To determine the significance of novel missense variations, we screened the allele frequency in 100 normal subjects and analyzed interspecies amino acid conservation using ClustalW. The mutation nomenclature followed the recommendations of the Human Genome Variation Society. Informed consent was obtained from the parents of each patient for genetic study of blood samples. Urine organic acid was measured by gas chromatography and a mass selective detector.
Although the pathogenic mechanism of specific central nervous system injury has not been established well, clinical and laboratory features have been well characterized recently from international cross-sectional study in 35 metabolic centers (2). Most of the symptomatic patients (185/218, 85%), had an acute encephalopathic crisis defined by acute neurological deterioration associated with infection or seizure. The study also pointed out that only limited patients developed neurologic deterioration without a reported crisis. Compared to these typical clinical courses, we found several different clinical features in our patients. Patient 1 had already showed delayed development and abnormal signals in both basal ganglia on MRI before acute encephalopathic crisis, suggesting that there was a preexisting injury before acute encephalopathic crisis. Patient 2 did not seem to have an overt acute encephalopathic crisis during the disease course. Sensory neural hearing loss, which was not reported to be an associated findings with GA I, was present before we diagnosed GA I. We performed surgical procedures in both patients before a diagnosis of GA I. We supposed chronic subdural hematoma or fluid collection partly contributed to delayed development in both patients. Interestingly, patient 2 showed much improvement in development after the surgical procedure, while patient 1 experienced no benefit but more deterioration. Asymmetric subdural fluid collection in patient 2 might be responsible for sub-acute neurological deterioration, considering the fact that patient 2 showed near normal neurodevelopment before deterioration. Recently published management guidelines of GA I recommended that neurosurgical intervention for subdural hemorrhage should be decided cautiously and conducted in limited, acute, life threatening circumstances including considerable mass effect or increased pressure (4). However, surgical intervention could play some beneficial role in carefully selected patients in addition to life threatening conditions, if acute or subacute deterioration of neurological status is suspected to be associated with subdural hematoma or fluid collection from clinical ground.
Our patients had compound heterozygote mutations in the GCDH. Patient 1 had a G-to-T transition (c.658G>T) in exon 8 (p.Asp220Tyr) and the other allele mutation was a C-to-T transition (c.416C>T) in exon 6 (p.Ser139Leu), which was also found in patient 2. A C-to-T transition (c.478C>T) in exon 6 (p.Glu160X) was the other mutation found in the patient 2. Among these, p.Glu160X and p.Asp220Tyr are novel mutations. They are thought to be disease-causing mutations because one is a nonsense mutation and the amino acid change caused by the other mutation is highly conservative, and we could not find this missense mutation in 200 alleles of normal controls. The most frequently found mutation in the European population is known to be p.Arg402Trp ranging from 16% to 40% (6). In Hong Kong and Tawian Chinese, IVS 10-2AC is the recurrent mutation (7, 8). Three point mutations including two novel mutations (p.Ser305Leu, p.Met-339Val, p.Arg355His) were reported in two Japanese patients (9). Although, the common mutation of our patients, p.Ser-139Leu, was first reported in 1998 from Western population (10), few reports have discussed the biochemical or clinical phenotype of this mutation after that. To date, two Korean GA I patients have been reported, diagnosed by urine organic acid analysis and typical brain MRI findings in Korean literature (11, 12).
In conclusion, we have described two patients with GA I, who displayed minor clinical differences to previous reports, proven by mutational analysis of the GCDH, for the first time in Korea. More GA I patients are expected to be diagnosed before acute encephalopathic crisis with more widespread use of MRI and newborn screening by tandem mass spectrometry. In these circumstances, mutation analysis will have a particularly important role for confirming the diagnosis of GA I. We hope, from the present study, more GA I patients would be diagnosed in the early course of disease and the genetic and clinical characteristics of GA I in the Korean population would be further clarified.
Figures and Tables
Fig. 1
Magnetic resonance imaging (MRI) findings and GCDH gene mutational analysis of two patients. (A) MRI of the patient 1. A T2-weighted axial image showing a large amount of subdural fluid collection with frontotemporal atrophy and high signal intensity in both basal ganglia. (B) A novel mutation from the patient 1. c.658G>T, heterozygote, p.Asp220Tyr. (C) MRI of the patient 2. Asymmetric subdural fluid collection suggesting hemorrhage with mild mass effect on a T2-weighted axial image. (D) A novel mutation from the patient 2. c.478C>T, heterozygote, p.Q160X.
References
1. Hedlund GL, Longo N, Pasquali M. Glutaric acidemia type 1. Am J Med Genet C Semin Med Genet. 2006. 142 C:86–94.
2. Kolker S, Garbade SF, Greenberg CR, Leonard JV, Saudubray JM, Ribes A, Kalkanoglu HS, Lund AM, Merinero B, Wajner M, Troncoso M, Williams M, Walter JH, Campistol J, Martí-Herrero M, Caswill M, Burlina AB, Lagler F, Maier EM, Schwahn B, Tokatli A, Dursun A, Coskun T, Chalmers RA, Koeller DM, Zschocke J, Christensen E, Burgard P, Hoffmann GF. Natural history, outcome, and treatment efficacy in children and adults with glutaryl-CoA dehydrogenase deficiency. Pediatr Res. 2006. 59:840–847.
3. Goodman SI, Markey SP, Moe PG, Miles BS, Teng CC. Glutaric aciduria; a "new" disorder of amino acid metabolism. Biochem Med. 1975. 12:12–21.
4. Kolker S, Christensen E, Leonard JV, Greenberg CR, Burlina AB, Burlina AP, Dixon M, Duran M, Goodman SI, Koeller DM, Müller E, Naughten ER, Neumaier-Probst E, Okun JG, Kyllerman M, Surtees RA, Wilcken B, Hoffmann GF, Burgard P. Guideline for the diagnosis and management of glutaryl-CoA dehydrogenase deficiency (glutaric aciduria type I). J Inherit Metab Dis. 2007. 30:5–22.
5. Lindner M, Kolker S, Schulze A, Christensen E, Greenberg CR, Hoffmann GF. Neonatal screening for glutaryl-CoA dehydrogenase deficiency. J Inherit Metab Dis. 2004. 27:851–859.
6. Christensen E, Ribes A, Merinero B, Zschocke J. Correlation of genotype and phenotype in glutaryl-CoA dehydrogenase deficiency. J Inherit Metab Dis. 2004. 27:861–868.
7. Tang NL, Hui J, Law LK, Lam YY, Chan KY, Yeung WL, Chan AY, Cheung KL, Fok TF. Recurrent and novel mutations of GCDH gene in Chinese glutaric acidemia type I families. Hum Mutat. 2000. 16:446.
8. Shu SG, Tsai CR, Chen LH, Chi CS. Type I glutaric aciduria: phenotypes and genotypes in 5 Taiwanese children. J Formos Med Assoc. 2003. 102:729–732.
9. Ikeda H, Kimura T, Ikegami T, Kato M, Matsunaga A, Yokoyama S, Yamaguchi S, Ohura T, Hayasaka K. Novel mutations of the glutaryl-CoA dehydrogenase gene in two Japanese patients with glutaric aciduria type I. Am J Med Genet. 1998. 80:327–329.
10. Goodman SI, Stein DE, Schlesinger S, Christensen E, Schwartz M, Greenberg CR, Elpeleg ON. Glutaryl-CoA dehydrogenase mutations in glutaric acidemia (type I): review and report of thirty novel mutations. Hum Mutat. 1998. 12:141–144.
11. Shin WJ, Moon YO, Yoon HR, Dong ES, Ahn YM. A case of glutaric aciduria type I with macrocephaly. J Korean Pediatr Soc. 2003. 46:295–301.
12. Song JY, Kim CM, Shin YL, Yoo HW. A case of glutaric aciduria type 1. J Korean Pediatr Soc. 2002. 45:1278–1282.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download