Abstract
It is unclear whether emphysema, regardless of airflow limitation, is a predictive factor associated with survival after lung cancer resection. Therefore, we investigated whether emphysema was a risk factor associated with the outcome after resection for lung cancer. This study enrolled 237 patients with non small cell lung cancer with stage I or II who had surgical removal. Patient outcome was analyzed based on emphysema. Emphysema was found in 43.4% of all patients. Patients with emphysema were predominantly men and smokers, and had a lower body mass index than the patients without emphysema. The patients without emphysema (n=133) survived longer (mean 51.2±3.0 vs. 40.6±3.1 months, P=0.042) than those with emphysema (n=104). The univariate analysis showed a younger age, higher FEV1/FVC, higher body mass index, cancer stage I, and a lower emphysema score were significant predictors of better survival. The multivariate analysis revealed a younger age, higher body mass index, and cancer stage I were independent parameters associated with better survival, however, emphysema was not. This study suggests that unfavorable outcomes after surgical resection of lung cancer should not be attributed to emphysema itself.
Lung cancer is the leading cause of death in cancer related mortality (1, 2). Complete surgical resection at an early stage is the only possible curative option for the treatment of lung cancer. Age, smoking, stage, and underlying lung function have been reported to be associated with the prognosis of patients after resection for lung cancer (3-5). However, even though lung cancer might be detected at an early stage, co-morbidities combined with the lung cancer often prevent patients from undergoing a surgical resection. Chronic obstructive pulmonary disease (COPD) is often diagnosed in patients with lung cancer, because COPD and lung cancer are mainly caused by smoking. COPD traditionally includes chronic bronchitis characterized by airflow limitation and emphysema with alveolar wall destruction. Emphysema is often detected even with no airflow limitation when chest tomography is performed for evaluations of lung cancer.
In some patients with lung cancer, there is a concern that the operative morbidity and mortality may be affected by the presence of emphysema; this may cause surgeons to be reluctant to subject patients to surgical treatment. The safety of surgical resections in patients with emphysema has not been resolved and the impact of emphysema on surgical outcome has not been clearly defined in patients with early lung cancer.
Therefore, the goal of this study was to investigate whether emphysema is a risk factor associated with the outcome of surgical resection in patients with early lung cancer.
This study enrolled 237 consecutive patients with stage I or II non-small cell lung cancer (NSCLC) that underwent surgical resection from March 2003 to July 2007 at Ajou Medical Center (a university affiliated 1000-bed sized tertiary referral center in Suwon, Korea). The decision to perform a surgical resection for the patients with lung cancer was made through discussion in the joint conference where pulmonologist, thoracic surgeon, oncologist, radiation oncologist, and radiologist participated. Age, FEV1, performance, and staging were the main factors in the decision regarding surgical resection.
The data were collected and analyzed retrospectively. All patients were followed in the outpatient clinic at three to six month intervals during the first three years after surgery. For the patients who are lost during follow up, telephone interview had us get the information on the patient's survival.
This study was approved by the Institutional Review Board of Ajou University Hospital (Approval number: AJIRB-CRO-08169).
The diagnosis of lung cancer was made using various methods, for example, sputum cytology, thoracentesis, fine needle aspiration, or bronchoscopy, as dictated by the patient's presentation. Pathologists interpreted the cytology or histology of tissue biopsy. The lung cancer was staged using a widely used classification system (6, 7) and the staging procedure included a clinical examination, standard chest radiography, computed tomography (CT) of the chest, abdomen, and brain, bronchoscopy, abdominal ultrasonography, bone scanning, and positron emission tomography (PET). For precise staging, only patients in whom pathologic staging was possible due to resection surgery were selected.
The diagnosis of COPD was determined by clinical criteria and previously documented airflow limitation (FEV1 <80% of the predicted value in combination with an FEV1/FVC <70% that is not fully reversible (8, 9). The severity of COPD was dictated by GOLD criteria (9). Spirometry was performed according to American thoracic society guidelines within 1 month before resection was performed (10).
Two radiologists who were blinded to the clinical informations determined the presence of emphysema and emphysema score by reviewing the thin-section CT scans.
All examinations were performed at our medical center using a 16-detector-row CT scanner. The CT examinations included pre and post-contrast scanning. The scanning parameters were 120 kVp and 70-200 mA. In all individuals, the scanning was performed during breath-holding at end inspiration. The thin-section CT scans were obtained with reconstruction thickness 1-1.2 mm using a high-frequency algorithm. When post-contrast scans were performed, a single phase contrast enhancement study was obtained after intravenous injection of nonionic contrast media (2.5 mL/sec, total of 100 mL of Iomeprol [Iomeron 300]; Bracco, Milan, Italy) using a power injector with a fixed delay time of 45 sec. The image data were reconstructed using a lung filter kernel at a slice thickness setting of 4 mm, at a 4-mm reconstruction increment.
total of five levels (6 and 3 cm above carina; carina; and 3 and 6 cm below carina) were evaluated using thin-section scanning to score the severity and extent of emphysema; two radiologists assessed the findings using direct observational methods (11-13). When disagreements occurred, a consensus was achieved. Severity was graded according to a 4 point scale as follows: 0, no emphysema; 1, all low-attenuation areas smaller than 5 mm in diameter; 2, all circumscribed low-attenuation areas larger than 5 mm in diameter in addition to those circumscribed areas smaller than 5 mm in diameter; 3, diffuse low-attenuation areas without intervening healthy lung or large, confluent low-attenuation areas. The extent of emphysema was also graded using a 4 point scale as follows: 1, less than 25% lung involvement; 2, 25-50% lung involvement; 3, 50-75% lung involvement; 4, more than 75% lung involvement. For each of the 10 lung sections, the score for the respective section was obtained by multiplying the severity of emphysema by the extent of emphysema score. The total score, ranging from 0 to 120, was obtained by adding each score for the 10 lung sections.
Finally, the patients with emphysema were grouped into four subgroups by the cumulative frequency scores: mild emphysema (Grade 1; emphysema scoring 1-10), moderate emphysema (Grade 2; 11-16), severe emphysema (Grade 3; 17-31), and very severe emphysema (Grade 3; 32-101).
SPSS version 12 (SPSS Inc., Chicago, IL, USA) was used for the analysis. All values are given as means±standard deviation, except for the survival period in which the mean±standard error was used. The values did not fit a standard distribution, so nonparametric analysis was performed. Survival curves were plotted using the Kaplan-Meier method, and the significance of differences between groups was analyzed using the log-ranks test. A Cox proportional hazards model multivariate analysis was used to evaluate factors contributing to survival. A value of P<0.05 was considered statistically significant.
The subjects were 237 consecutive patients (189 males and 48 females, mean age: 63.0±10.6 yr) that had NSCLC surgically removed. Emphysema was found in 43.4% of all patients with lung cancer. After surgical resection of the lung cancer, 67.1% of all patients were confirmed to have stage I disease.
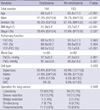
The emphysema patients were older and had a lower body mass index (BMI) compared to the patients without emphysema. Smokers were predominant in the emphysema group (90.3% vs. 67.4%; P<0.001). Accordingly, squamous cell carcinoma, which occurs mostly in smokers, was more frequent in patients with emphysema; adenocarcinoma was more common in the patients without emphysema (Table 1).
Airway obstruction represented by the FEV1 (80.9±20.7% vs. 88.8±20.8%; P=0.004) and FEV1/FVC (69.5±13.27% vs. 76.1±9.8% P<0.001) was worse in the patients with emphysema than in those without emphysema. However, there was no significant difference in the blood gas findings between the two groups (Table 1).
For the patients with emphysema, the hospital stay tended to be longer compared to the patients without emphysema. However, there was no significant difference in the duration of intensive care unit (ICU) stay or chest tube placement after surgical resection (Table 2).
The mortality after surgical resection at 30 days was 2.9% in all patients with lung cancer, and the mortality at 90 days was 7.6%. However, there was no significant difference in the mortality rate at 1 month or 3 months based on the presence of emphysema. In addition, the complication rate was not different between the two groups. The most common postoperative complication was pneumonia, which occurred in 12.5% of the patients with emphysema and in 9.0% of the patients without emphysema (Table 2).
Using the log rank test, the survival curves were compared between patients with and without emphysema. In all patients, the patients without emphysema (n=133, mean: 51.2±3.0 months) survived longer than those with emphysema (n=104, mean: 40.6±3.1 months, P=0.042) (Fig. 1). Likewise, for stage I lung cancer, patients without emphysema survived longer (n=97, mean: 57.9±3.1 months) than those with emphysema (n=62, mean: 44.0±3.9 months, P=0.036) (Fig. 2). In addition, in selected patients with stage I lung cancer and a normal FEV1 (FEV1 ≥80%), patients without emphysema survived slightly longer (n=68, mean: 55.4±2.9 months) than those with emphysema (n=32, mean: 46.6±5.3 months, P=0.076).
When survival was analyzed by grouping patients based on a 25% cumulative frequency of the emphysema scoring, only the patients with very severe emphysema survived for a far shorter period than the other three subgroups (Fig. 3). The Cox regression analysis was used to analyze the association of emphysema and other factors to patient survival. According to the univariate Cox model, a younger age, higher FEV1/FVC, higher BMI, cancer stage, and lower emphysema score were significantly associated with a better survival (Table 3). The multivariate analysis of all patients revealed that a younger age, higher BMI, and stage I disease were independent parameters associated with a better survival; however, emphysema per se and the emphysema grade were not independent factors associated with survival (Table 4).
The results of our study demonstrated that in patients with early resected NSCLC (stage I and II), emphysema was not an independent parameter associated with survival after the resection of lung cancer. The findings showed that patients with emphysema had longer hospital stay and shorter survival on the univariate analysis. Patients without emphysema survived longer than those with emphysema after surgical resection of lung cancer, even in selected patients with cancer stage I. After lung cancer resection, the survival time of a subgroup with very severe emphysema was shorter than in the other three subgroups (no, mild, moderate, and severe emphysemas), when survival was analyzed by grouping patients based on a 25% cumulative frequency of the emphysema scoring.
However, the results of the multivariate analysis showed that age, cancer stage, and BMI were independent factors associated with survival after the resection of lung cancer, whereas emphysema per se was not a significant factor nor was the emphysema scoring. This discrepancy between the univariate and multivariate analysis might be attributed to the demographic data showing older smokers and diminished pulmonary function in the patients with emphysema. These confounding factors might have affected the prognosis after lung cancer resection and should be adjusted by the multivariate analysis, as shown in our data.
Patients with NSCLC have a higher prevalence rate of co-morbidity associated with age and smoking. This co-morbidity can have a significant impact on survival after surgical resection of patients with early NSCLC (14, 15). Therefore decisions to proceed with surgery in such patients should be made on a case by case basis weighing the clinical benefits and risks based on the significant associated outcome factors reported in this study. However, the findings of this study should reassure clinicians with regard to surgical therapy in early NSCLC patients that have emphysema.
When we confront the patients with severe emphysema who are about to undergo surgical resection, authors' opinion is that clinicians should be alerted more to risk factors including age, cancer stage, BMI, and pulmonary function test which may possibly have an influence on the prognosis after surgery than emphysema itself because severe emphysema can be accompanied with many co-morbidities.
NSCLC patients up to stage II, determined by surgical resection, were enrolled in our study. These patients were chosen because of the conflicting opinions on the necessity of surgery in the patients with stage IIIa lung cancer (16, 17).
Airway obstruction represented by the FEV1, the most important factor for patient prognosis after surgery, was not a crucial factor in this study. This finding is likely explained by the fact that patients with severe airway obstruction were not considered candidates for surgery.
BMI, has been shown to be an independent factor in the prognosis of patients with COPD; therefore nutritional care seems to be important for these patients (18, 19). The results of this study showed that a lower BMI had a significant impact on survival after surgical resection. Therefore, it is thought that BMI should be considered an additional risk factor for surgery in patients with lung cancer.
COPD traditionally includes chronic bronchitis characterized by airflow limitation and emphysema with alveolar wall destruction. Because emphysema is often present without airflow limitation detected by pulmonary function testing, emphysema can go unnoticed unless a chest CT reveals lung parenchymal destruction. Therefore, emphysema might be more frequently found in patients with lung cancer because thorough evaluations including chest CT are usually performed. Emphysema can even be an independent risk factor for the development of lung cancer (20). Our data showed that 43.4% of patients with lung cancer had emphysema. One retrospective study reported that twelve percent of all cancer patients had COPD at the time of the cancer diagnosis and COPD was associated with reduced survival in a variety of cancers including breast, prostate, and urinary bladder cancer in middle aged patients, and in colon, larynx, prostate, and urinary bladder cancer in elderly patients (21). However, the impact of emphysema on the outcome of surgery for lung cancer continues to be debated. Ueda et al. reported that emphysema diagnosed by computed tomography was associated with a poor prognosis after surgical resection in patients with lung cancer (22). Their study is supported by theories suggesting that emphysema may reflect an increased susceptibility to smoking related biological damage, which ultimately determines the aggressiveness of tumor cells (23). In addition, tumor progression may be enhanced in emphysematous lungs where metalloproteinases are abundant (24). However, conflicting opinions have also existed. Patients with resectable lung cancer and emphysema may have a good long term survival after cancer resection because cancer resection provides a volume reduction effect (25). Patients with severe pulmonary dysfunction have had volume reduction surgery and pulmonary nodule resection, with favorable outcomes (26).
Previous studies have reported that postoperative ventilatory function, in patients with COPD, were well preserved after lobectomy (27, 28). We did not have the information needed to report on a volume reduction effect in this study, as acknowledged in the limitations below.
We acknowledge several limitations of this study. First, DLCO, which is associated with the degree of emphysema and prognosis after surgery, was not analyzed due to the retrospective design. Likewise, follow up by pulmonary function test was not conducted in a well-controlled manner, which might keep us from assessing postoperative condition exactly. Second, the etiology of emphysema such as antitrypsin deficiency was not determined because this disease is rare in Korean society. Third, a selection bias could have been present due to the fact that most patients resided in an area around Suwon city. Therefore, the patients in our study might not represent the Korean population. Fourth, decisions regarding surgery were determined by consensus of the thoracic surgeon, pulmonologist and radiologist; only cases that had the stage confirmed pathologically were enrolled. However, the possibility of surgeon's bias in selecting the candidate for resection of lung cancer cannot be ruled out and might have affected our data.
In conclusion, the results of this study suggest that age, cancer stage, and BMI were independent factors associated with survival after resection of the lung cancer, whereas emphysema per se was not. Therefore, unfavorable outcomes after surgical resection should not be attributed to emphysema itself.
Figures and Tables
Fig. 1
Kaplan-Meier curves for Survival of total Patients depending on the presence of emphysema (○emphysema (n=104), mean survival, 40.6±3.1 months; •non emphysema (n=133) mean survival, 51.2±3.0 months, P=0.042).

Fig. 2
Kaplan-Meier curves for survival of NSCLC patients with stage I depending on the presence of emphysema (○emphysema (n=62), mean survival, 44.0±3.9 months; •non emphysema (n=97), mean survival, 57.9±3.1 months, P=0.036).

Fig. 3
Survival of NSCLC Patients based on the degree of emphysema (Mean survival period of each group; Emp 0 (Grade 0, n=133), 51.2±3.0 months; Emp 1 (Grade 1, n=19), 41.7±7.6 months; Emp 2 (Grade 2, n=21), 41.1±5.7 months; Emp 3 (Grade 3, n=20), 44.2±6.8 months; Emp 4 (Grade 4, n=20), 26.3±7.1, P=0.07). Emp, emphysema.

References
2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.

3. Reilly JJ. Preparing for pulmonary resection: preoperative evaluation of patients. Chest. 1997. 112:4 Suppl. S206–S208.
4. Colice GL, Shafazand S, Griffin JP, Keenan R, Bolliger CT. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007. 132:3 Suppl. S161–S177.
5. British Thoracic Society. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001. 56:89–108.
6. Mountain CF. A new international staging system for lung cancer. Chest. 1986. 89:4 Suppl. S225–S233.

7. Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997. 111:1710–1717.

8. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987. 136:225–244.
9. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007. 176:532–555.
10. American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med. 1995. 152:1107–1136.
11. Sakai F, Gamsu G, Im JG, Ray CS. Pulmonary function abnormalities in patients with CT-determined emphysema. J Comput Assist Tomogr. 1987. 11:963–968.

12. Klein JS, Gamsu G, Webb WR, Golden JA, Muller NL. High-resolution CT diagnosis of emphysema in symptomatic patients with normal chest radiographs and isolated low diffusing capacity. Radiology. 1992. 182:817–821.

13. Lamers RJ, Thelissen GR, Kessels AG, Wouters EF, van Engelshoven JM. Chronic obstructive pulmonary disease: evaluation with spirometrically controlled CT lung densitometry. Radiology. 1994. 193:109–113.

14. Moro-Sibilot D, Aubert A, Diab S, Lantuejoul S, Fourneret P, Brambilla E, Brambilla C, Brichon PY. Comorbidities and Charlson score in resected stage I nonsmall cell lung cancer. Eur Respir J. 2005. 26:480–486.

15. Birim O, Kappetein AP, Bogers AJ. Charlson comorbidity index as a predictor of long-term outcome after surgery for nonsmall cell lung cancer. Eur J Cardiothorac Surg. 2005. 28:759–762.

16. Whitson BA, Groth SS, Maddaus MA. Surgical assessment and intraoperative management of mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg. 2007. 84:1059–1065.

17. Kang CH, Ra YJ, Kim YT, Jheon SH, Sung SW, Kim JH. The impact of multiple metastatic nodal stations on survival in patients with resectable N1 and N2 nonsmall-cell lung cancer. Ann Thorac Surg. 2008. 86:1092–1097.

18. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004. 350:1005–1012.

19. Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sorensen TI, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006. 173:79–83.

20. Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, Sciurba FC. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008. 178:738–744.

21. van de Schans SA, Janssen-Heijnen ML, Biesma B, Smeenk FW, van de Poll-Franse LV, Seynaeve C, Coebergh JW. COPD in cancer patients: higher prevalence in the elderly, a different treatment strategy in case of primary tumours above the diaphragm, and a worse overall survival in the elderly patient. Eur J Cancer. 2007. 43:2194–2202.

22. Ueda K, Jinbo M, Li TS, Yagi T, Suga K, Hamano K. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res. 2006. 12:6730–6736.

23. Finlay GA, O'Driscoll LR, Russell KJ, D'Arcy EM, Masterson JB, FitzGerald MX, O'Connor CM. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med. 1997. 156:240–247.

24. Ishikawa S, Takenaka K, Yanagihara K, Miyahara R, Kawano Y, Otake Y, Hasegawa S, Wada H, Tanaka F. Matrix metalloproteinase-2 status in stromal fibroblasts, not in tumor cells, is a significant prognostic factor in non-small-cell lung cancer. Clin Cancer Res. 2004. 10:6579–6585.

25. Choong CK, Meyers BF, Battafarano RJ, Guthrie TJ, Davis GE, Patterson GA, Cooper JD. Lung cancer resection combined with lung volume reduction in patients with severe emphysema. J Thorac Cardiovasc Surg. 2004. 127:1323–1331.

26. Vaughan P, Oey I, Nakas A, Martin-Ucar A, Edwards J, Waller D. Is there a role for therapeutic lobectomy for emphysema? Eur J Cardiothorac Surg. 2007. 31:486–490.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download