Abstract
Hemosiderin deposition is not often recognized on routine examination with hematoxylin and eosin staining; however, iron stains may be helpful in the evaluation of hemosiderin deposition in infant autopsies. This report describes the data obtained from autopsy of 86 infants and children whose deaths were investigated at the Forensic Medicine Council Bursa Morgue Department from January 2000 to January 2003. A histochemical technique was used to identify hemosiderin in lung, liver and spleen specimens, which was correlated with other descriptive variables such as the reported cause of death, postmortem interval, trauma history, gender, and age. There was a weakly positive but significant correlation between lung and liver hemosiderin scores (Spearman's rank correlation coefficient, rho=0.348, p=0.001); i.e., given an increase in lung hemosiderin scores, an increase in liver hemosiderin scores was also observed. Similarly, a marked positive correlation between spleen and liver hemosiderin scores (Spearman's rank correlation coefficient, rho=0.335, p=0.002) was observed. The probability of spleen hemosiderin-positive cases belonging to the age group under 6 months was found to be 4.3 times greater than those who were hemosiderin-negative (95% confidence interval, 1.6-11.8). After the major differential diagnoses were ruled out, this study demonstrated, that depending on the statistically assessed morphometric grounds, the presence of hemosiderin deposits in the liver and spleen were significantly higher in the age group under 6 months.
If hemosiderin is present in increased amounts without an obvious explanation, further investigation is warranted. Research on hemosiderin deposits in autopsies of organs such as the lungs, liver and spleen has not been conducted as much as that on deposits in skin, brain and soft tissue (1, 2). Indeed, it is far more common to study hemosiderin deposits in the latter organs (3). One issue that arises in post-mortem studies of infants is the amount and significance of hemosiderin found in infant lungs; furthermore, depositions in the liver and spleen are not well defined (1, 4, 5). Also, in the context of traumatic injuries like chronic physical child abuse, histological examinations for the presence of hemosiderin deposits are necessary to look for sequelae of repeated hemorrhages (4), particularly in organs likely to have suffered from trauma, such as the lungs, or in organs belonging to the mononucleated macrophage resorption system, such as the liver and the spleen (6). Hemosiderin has also been reported as a marker of a previous asphyxic event, and possible grounds for suspicion of homicide in infant deaths (2, 6, 7), but a review of the available literature does not provide a strong evidence to support this claim. Iron-laden macrophages and hemosiderin deposition are often not recognized on routine examination with hematoxylin and eosin (H & E) staining; thus, iron stains may be helpful in the evaluation of infant deaths (1, 2). In order to evaluate this hypothesis, we looked for microscopical evidence of hemosiderin depositions in organs likely to have suffered from trauma, such as the lungs, or organs belonging to the mononucleated macrophage resorption system, such as the liver and the spleen.
Infant and child lung samples were obtained prospectively at autopsy by forensic medical examiners in the Forensic Medicine Council Bursa Morgue Department. The tissues were submitted regardless of the cause of death. Eighty-six cases were evaluated for the study. Two independent medical examiner pathologists participated in the study. The sections were examined by two pathologists who were blinded to the study details. Complete autopsies, including toxicological and histopathological analysis, were performed by forensic examiners and pathologists. We examined two randomly selected specimens from the lungs and one each from the liver and the spleen. The samples were fixed in a 10% formalin solution for at least 5 days, embedded in paraffin wax, cut into 5 mm sections and stained with H & E for microscopic examination. Hemosiderin deposits were detected using a Prussian blue histochemical technique (Perls's stain for iron to generate ferric ferrocyanate, allowing identification of ferric ions [Fe+++]). For this staining series, we used a solution of 100 mL 2% hydrochloric acid and 100 mL 2% potassium ferrocyanide. This report presents baseline data for lung, liver, and spleen hemosiderin content among 86 infants regardless of the cause of death. The Golde score is largely used in pathology for the quantification of lung hemosiderin deposits, and the Deugnier score is used for the quantification of liver hemosiderin deposits; these are considered to be reliable and reproducible. We used Hanzlick's method for lung iron examination (1), and also applied it to the liver and spleen. Two pathologists independently microscopically scanned the lung, liver, spleen sections using a 10× objective lens (with a 10× ocular lens), and determined the iron score for each section: 0, if it showed no staining; 1, occasional staining with most fields negative; 2, focally abundant staining with most fields having no staining; 3, focally abundant staining with most fields showing positive staining; or 4, prominent staining throughout the section score. A total iron score for each organ was calculated by adding the scores based on each pathologist's observation. In all of the cases, sufficient information was available to calculate the interval between death (or the infant being found dead) and performance of the autopsy. The results were subsequently correlated to case history, autopsy findings, gender, cause of death, and traumatic autopsy findings like ecchymoses, bruises and postmortem interval (PMI).
A complete analysis was performed using SPSS 11.0 version for Windows (SPSS Inc., Chicago, IL, U.S.A.). A total of 86 subjects were analyzed. Continuous variables were presented as mean±standard deviation (SD) and median. Categorical variables were presented as frequencies (N, %). The Pearson chi-square test and Kolmogorov-Smirnov test were used to compare categorical variables. The Kruskal-Wallis test and Mann-Whitney U test were used to compare the means between groups. Kappa statistics were used as a measure of agreement between the independent qualitative interpretations of the two investigators. Interobserver agreement was considered excellent if the Cohen's kappa value was between 0.90 and 1.0. Correlations were evaluated by Spearman's correlation analysis. Determination of the prediction factors for the presence of lung, liver and spleen hemosiderin deposits was estimated by multivariate logistic regression analysis (forward stepwise). All statistical analyses were performed according to two-sided hypothesis tests, and a p value of less than 0.05 was regarded as statistically significant.
Eighty-six cases were reviewed (Table 1). The gross examination of the visceral organs did not disclose any significant abnormalities. The lungs, spleens and livers had weights within the normal limits. Among all of the cases, only 13 had total lung iron scores that were at least half of the maximum score (i.e., a total lung iron score of 8 or higher); from these cases, eight had total liver iron scores that were at least half of the maximum liver iron score (i.e., a total liver iron score of 4 or higher), and 3 had total spleen iron scores that were at least half of the maximum spleen iron score (i.e., a total spleen iron score of 4 or higher). In 11 of these cases, the cause of death was not sudden unexpected death (SUD), and these included cases of congenital heart disease, myocarditis, myocardial infarction, bronchopneumonia and trauma. Of the 14 SUD cases, only six had total lung iron scores that were at least half of the maximum score; six had at least half of maximum liver iron score and four had at least half of the maximum spleen iron score. Among the 16 pneumonia cases, eight cases had total lung iron scores that had at least half of the maximum score; 7 cases had at least half of the maximum liver iron score and 3 cases had at least half of the maximum spleen iron score. There were 12 cases with an undetermined cause of death, only three of which had total lung iron scores that were greater than half of the maximum score; one case had at least half of the maximum liver iron score and two had at least half of the maximum spleen iron score.
There was an excellent interobserver agreement between the reviewing pathologists on category assignment regarding the two lung specimens (kappa=0.924 and kappa=1.0; p<0.001), liver hemosiderin scores (kappa=0.924, p<0.001) and spleen iron stain score (kappa=0.951, p<0.001). Positive lung iron staining was found primarily within macrophages in all hemosiderin-positive cases (Fig. 1).
In all groups, the total lung hemosiderin deposition scores did not markedly differ according to gender, age or cause of death (p>0.05). Thirty-five of the 56 (62.5%) liver hemosiderin-positive cases and 6 of the 30 (20%) liver hemosiderin-negative cases belonged to the under 6-month age group (p<0.001). The probability of liver hemosiderin-positive cases being in the under 6-month age group was found to be 6.7 times greater than that of those who were hemosiderin-negative (odds ratio [OR], 6.7, 95% confidence interval [CI], 2.3-18.9) (Fig. 2). Similarly, 18 of the 25 (72%) spleen hemosiderin-positive cases and 23 of the 61 (37.7%) spleen hemosiderin-negative cases were in the under 6-month age group (p=0.004). The probability of spleen hemosiderin-positive cases being in the under 6-month age group was found to be 4.3 times greater than that of those who were hemosiderin-negative (OR, 4.3, 95% CI, 1.6-11.8) (Fig. 2).
When the cases were grouped according the manner of death, natural death (N=60), unnatural death (N=14), or undetermined (N=12), 11 of 14 (68%) unnatural death cases and all of the undetermined cases were in the older than 6-month age group and 38 of 60 (63.3%) natural death cases were in the under 6-month age group (p<0.001). In 5 of 14 (68%) unnatural death cases, 7 of 12 (58.3%) undetermined cases and 54 of 60 (90%) natural death cases, hemosiderin presence was detected at least in one of the organs investigated (p=0.027).
In univariate analysis, the probability of lung hemosiderin positivity in the natural death group was found to be 5.1 times greater than that of those who were in the unnatural death group (OR, 5.133 95% CI, 1.297-20.321), and liver hemosiderin positivity in the natural death group was found to be 5.4 times greater than that of those who were in the unnatural death group (OR, 5.400, 95% CI, 1.563-18.653).
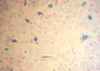
In our cases, liver hemosiderin deposits were most often
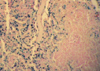
found both in hepatocytes and Kupffer cells (Fig. 3). The spleen showed a siderosis of the red pulp (Fig. 4).
There were statistically significant differences between trauma findings and age groups. The mean age of the 54 cases without trauma findings was 22.7±51.6 months (median, 4 months). Thirty-three of the 54 (61.1%) non-traumatic cases and 3 of the 17 (17.6%) traumatic cases were in the under 6-month age group (p=0.002). There was also a significant male predominance in the traumatic group; 15 of 17 (88.2%) traumatic cases were male and 29 of 54 (53.7%) non-traumatic cases were male (p=0.011). But there were no statistically significant differences between traumatic and non-traumatic groups according to lung, liver, or spleen hemosiderin deposition. There was a weakly positive but significant correlation between lung and liver hemosiderin scores as the lung hemosiderin scores increased (r=0.348, p=0.001). There was also a significant positive correlation between the spleen and liver hemosiderin scores (Spearman's correlation coefficient=0.335, p=0.002), with an increase in liver hemosiderin scores with an increase in spleen hemosiderin scores (Table 2). The median postmortem interval was 1 day (min.-max. =0-7 day). When the cases were divided into two groups based on PMI, those with PMI less than 24 hr or more than 24 hr, no significant differences were found between the liver and spleen hemosiderin scores (p>0.05), nor was there any significant correlation between them (p>0.05). Furthermore, there was a very weakly negative but statistically significant correlation between the lung hemosiderin scores and the postmortem interval (Spearman's correlation coefficient=-0.222, p=0.04) with an increase in postmortem interval there was a decrease in the lung hemosiderin scores (Table 2).
The determination of the predictors of hemosiderin deposition in each organ was estimated by multivariate logistic regression analysis. In a multivariate logistic regression analysis model, each organ's hemosiderin deposition (yes or no) and the presence of of hemosiderin in any organ were the dependent variables, and sex, age group, manner of death, traumatic findings and postmortem interval were the independent variables. The presence of trauma findings was found to significantly the odds of having spleen hemosiderin deposits (9.4 times). It was also found that being in the under 6-month age group significantly increased the odds of having liver hemosiderin deposits (Table 3).
Research on hemosiderin deposition in organs such as the lungs, liver and spleen has been less prevalent than that on deposits in the skin, brain and soft tissue (1, 2). Indeed, it has been far more common to study hemosiderin deposits in the latter organs (3). Hemosiderin in the lungs is a feature of primary idiopathic hemosiderosis, but in these cases, unlike ours, hemosiderin deposits were mostly found in interstitial macrophages (4). In infants, repeated pulmonary physical therapy and lung infections may also cause microscopical hemorrhages in the lungs (1). Iron supplementation, blood transfusions, erythroblastosis, hemolysis or coagulopathies of various types and fetal hydrops which can cause diffuse hemosiderin deposits in visceral organs, must be ruled out (4, 5).
The presence of hemosiderin deposits is evidence of chronic bleeding, and when it is found in organs likely to have suffered trauma or organs belonging to the macrophage resorption system, it may be suggestive of previous child abuse (4) or asphyxial episodes, whether idiopathic or intentional (6, 7). Cases of well-documented asphyxia have been described by some authors, and pulmonary macrophages and hemosiderin were suggested as markers for previous asphyxial episodes (1, 7), but in our asphyxial deaths, the iron scores were not significantly high and there were no repeating previous asphyxial episodes between cases (p>0.05). Milroy presented a case of Munchausen syndrome by Proxy where there were deliberate acts of repeated partial smothering, and claimed that the finding of intra-alveolar hemosiderin at autopsy provided additional supportive evidence of smothering (8). There is also evidence that some infant fatalities diagnosed as sudden infant death syndrome may actually be cases of pulmonary hemorrhage/hemosiderosis that were not initially recognized as such (9), because a pulmonary hemorrhage/hemosiderosis is a rare condition usually characterized by recurrent episodes of diffuse pulmonary hemorrhage occurring as an isolated acute hemorrhage, or may involve multiple episodes of hemorrhage with hemosiderin accumulation (1, 9). According to some authors, these two manifestations can sometimes be distinguished on the basis of the degree of hemosiderosis in the lungs (10). Becroft et al. found that pulmonary interstitial hemosiderin was not found in any of the 13 infants delivered by elective cesarean section, compared with a 33% incidence in those exposed to labor (p=0.02) (11). There was no association with previous apnea. The likely explanation for these findings is that pulmonary interstitial hemosiderin is a result of interstitial hemorrhage caused by chest compression during labor that occurs particularly in larger infants of greater gestational age, and then clears gradually during early infancy. In concert with our findings, Risse and Weiler reported that the hemosiderin content detected in the lung, liver and spleen depends on age and decreases with increasing age (12). No relevant differences were found in cases of sudden infant death syndrome, and they reported that minimal intrapleural, septal and perivasal depositions of hemosiderin can be frequently found in the lung tissue. We also detected hemosiderin deposits in the lung in 46.51% of the cases. Stewart and Fawcett found hemosiderin deposits without inflammatory changes in the lungs of 10 children from a series of 24 cases of sudden infant death (6). These changes were thought to be the consequence of hypoxemic episodes preceding death, the mechanism of which was not detailed. These authors did not describe the precise technique used to quantify the hemosiderin deposits, which makes it difficult to compare our results. Byard et al. also found that hemosiderin deposits were more abundant in the lungs of children who died from sudden infant death syndrome than in controls with known non-sudden infant death syndrome and non-traumatic causes of death (13). Lewis et al. reported 6 cases of sudden and unexpected death of infants older than 4 days with massive pulmonary hemorrhage and patent ductus arteriosus at autopsy (14). Histology samples were examined for the distribution of hemorrhage in the lungs and iron was stained for hemosiderin evaluation. All of the cases had clinical histories and scene examinations which raised the differential diagnosis of mechanical asphyxia in the form of so-called overlayings. They stated that there is potential association of PDA with massive pulmonary hemorrhage, which also explains the high scores in our cases with congenital heart diseases.
A higher-than-average total iron score might be expected in the cases of congenital heart disease and acute or chronic heart failure, which can cause secondary pulmonary disease in the form of pulmonary hemorrhage (from congestive failure) or pneumonia (which can involve macrophages or hemorrhage as parts of the inflammatory process), in concert with the report of Hanzlick and Delaney (1). On the other hand, Silver et al. investigated a characteristic tissue iron storage pattern in 15 neonatal autopsy cases of hydrops fetalis in which both the clinical and gross autopsy findings suggested intrauterine congestive heart failure (5). The researches stated that, histologic assessment of the pattern of iron storage helped for confirmation of the pathologic diagnosis of fetal hydrops, also claimed that, analysis of the pathologic findings led to a scheme for categorizing cardiogenic fetal hydrops. Pneumonia, which was present in 16 cases, is often accompanied by pulmonary hemorrhage, and could include a macrophage response if present for several days. The high lung iron scores of the pneumonia cases support this. Hemosiderin deposits in the liver may be a feature of neonatal idiopathic hemochromatosis, and may also be found in patients who have taken iron supplement tablets. Microscopic examination may disclose an angiomatosis of the liver or intrahepatic erythropoiesis; it may also confirm neonatal idiopathic hemochromatosis (15). Dorandeu et al. claimed that, as in our cases, liver hemosiderin deposits were most often found both hepatocytes and Kupffer cells, unlike in cases of hemochromatosis where they were only found in hepatocytes (4). Risse and Weiler reported that the liver hemosiderin is localized predominantly in the periphery of the lobules, and the spleen shows a diffuse siderosis of the red pulp, as in our cases (12). Chronic child abuse in the absence of characteristic macroscopical lesions represents a difficult problem in forensic practice (6). The child's environment and police investigations are usually decisive; it has been reported that the findings suggest that hemosiderin deposits in the lungs and liver may be a feature of chronic child abuse and should be looked for microscopically in cases of suspicious deaths of infants and young children (4). Dorandeu et al. examined a series of 15 young children who died from proven chronic child abuse and compared them with 15 sex- and age-matched control subjects who died from natural causes with no history of child abuse (4). Hemosiderin deposits were significantly (p<0.001) more abundant in the lungs and liver of the chronic abuse victims than in those of the control subjects. However, they were not significantly more abundant in the spleens of child abuse victims than in controls. It was proposed that hemosiderin deposits in the lungs and liver could be used as a marker for chronic physical child abuse. This study stresses the importance of a systematic histological examination to look for pulmonary and hepatic hemosiderin deposits in cases where chronic child abuse is suspected (4). In the univariate analysis of our study, there were no statistically meaningful associations between the lung, liver, and spleen hemosiderin deposits in the traumatic and non-traumatic findings groups. But we did find a significant correlation between the lung and liver (Spearman's correlation coefficient=0.348, p=0.001) and spleen and liver (Spearman's correlation coefficient=0.335, p=0.002), hemosiderin deposits; with an increase in the lung hemosiderin scores, there was increase in the liver hemosiderin scores, and with an increase in the liver hemosiderin scores, there was increase in the spleen hemosiderin scores. By the application of multivariate logistic regression analysis, the presence of trauma findings was found to significantly the odds of having spleen hemosiderin deposits (9.4 times). After acute pulmonary hemorrhage, pulmonary macrophages may become laden with hemosiderin and have been shown to take 24 to 48 hr to break down iron from hemoglobin in erythrocytes. It can take up to 2 weeks for the lungs to clear hemosiderin-laden macrophages after acute hemorrhage, which disappear between the second or third month after clearing by the macrophage resorption system (6, 16). However, further studies are needed to stress the importance of systematic histological examination to look for pulmonary, hepatic and splenic hemosiderin deposits in cases in which chronic child abuse is suspected. Hanzlick and Delaney (1) reported that cases with total lung iron scores at least twice the mean showed no distinct correlation with the interval between the onset of fatal events and the actual time of death, but in our study there was weakly negative but statistically meaningful correlation between the lung hemosiderin scores and the postmortem interval (Spearman's rank correlation coefficient, rho=-0.222, p=0.040); with an increase in the postmortem interval there was a decrease in lung hemosiderin scores. In the literature, it has been mentioned that in delayed postmortem examination, putrefactive gas may oxidize normal Fe, giving false-positive results (17). After the major differential diagnoses were ruled out, our study demonstrated that, on statistically assessed morphometric grounds, the presence of hemosiderin deposits in the liver and spleen were significantly greater in the under 6 month age group than in the older age group, which was very likely consequence of hemoglobin catabolism from old microscopic bleeding caused by trauma.
It seems crucial to extend this study and systematically examine the visceral organs of children who have died from proven chronic child abuse and also those of children who have died under suspicious circumstances.
Figures and Tables
Fig. 1
Lung iron staining was predominantly found within macrophages. Perls's stain, 40× magnification.
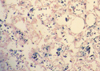
Fig. 2
Liver and spleen hemosiderin deposits in the under 6 months and older than 6 months of age groups.

Table 1
Descriptive information of the cases (N=86)

*, Other (drowning, electrocution, head trauma, hepatitis, meningitis, or poisoning); †, natural deaths (SUD, pneumonia, cardiac, metabolic, hepatitis, or meningitis); ‡, unnatural deaths (drowning, electrocution, head trauma, asphyxia, or poisoning); §, organs hemosiderin involvement (lung, liver, or spleen).
SUD, sudden unexpected death.
ACKNOWLEDGMENTS
The study was supported by the Forensic Medicine Council. The authors thank Dr. Filiz Yurdasen Eren for her investigation of pulmonary, liver and spleen hemosiderin deposition among infants. We also thank the Bursa Morgue Department of Council of Forensic Medicine, which funded the tissue processing, and the Laboratory of the Pathology Department of Bursa Morgue Department, which processed the tissues and performed the special histologic stains.
References
1. Hanzlick R, Delaney K. Pulmonary hemosiderin in deceased infants: baseline data for further study of infant mortality. Am J Forensic Med Pathol. 2000. 21:319–322.
2. Schluckebier DA, Cool CD, Henry TE, Martin A, Wahe JW. Pulmonary siderophages and unexpected infant death. Am J Forensic Med Pathol. 2002. 23:360–363.

3. Betz P, Eisenmenger W. Morphometrical analysis of hemosiderin deposits in relation to wound age. Int J Legal Med. 1996. 108:262–264.

4. Dorandeu A, Perie G, Jouan H, Leroy B, Gray F, Durigon M. Histological demonstration of haemosiderin deposits in lungs and liver from victims of chronic physical child abuse. Int J Legal Med. 1999. 112:280–286.

5. Silver MM, Perrin D, Smith CR, Freedom RM. Tissue iron storage patterns in fetal hydrops associated with congestive heart failure. Pediatr Pathol Lab Med. 1996. 16:563–582.

6. Stewart S, Fawcett J, Jacobson W. Interstitial haemosiderin in the lungs of sudden infant death syndrome: a histological hallmark of 'ear-miss' episodes? J Pathol. 1985. 145:53–58.
7. Becroft DM, Lockett BK. Intra-alveolar pulmonary siderophages in sudden infant death: a marker for previous imposed suffocation. Pathology. 1997. 29:60–63.

8. Milroy CM. Munchausen syndrome by proxy and intra-alveolar haemosiderin. Int J Legal Med. 1999. 112:309–312.

9. Centers for Disease Control and Prevention (CDC). Update: pulmonary hemorrhage/hemosiderosis among infants-Cleveland, 1993-1996. MMWR Morb Mortal Wkly Rep. 1997. 46:33–35.
11. Becroft DM, Thompson JM, Mitchell EA. Pulmonary interstitial hemosiderin in infancy: a common consequence of normal labor. Pediatr Dev Pathol. 2005. 8:448–452.

12. Risse M, Weiler G. Hemosiderin findings in the liver, spleen and lung in newborn infants and infants. Z Rechtsmed. 1987. 98:181–190.
13. Byard RW, Stewart WA, Telfer S, Beal SM. Assessment of pulmonary and intrathymic hemosiderin deposition in sudden infant death syndrome. Pediatr Pathol Lab Med. 1997. 17:275–282.

14. Lewis MJ, McKeever PK, Rutty GN. Patent ductus arteriosus as a natural cause of pulmonary hemorrhage in infants: a medicolegal dilemma. Am J Forensic Med Pathol. 2004. 25:200–204.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download