Abstract
Numerous studies have demonstrated the clinical activity of temozolomide, a second-generation alkylating agent, against malignant brain tumors, however, its activity has not been reported in an Asian population. This study analyzed the efficacy and toxicity of temozolomide in 25 adult patients with recurrent or progressive malignant gliomas after surgery and standard radiation therapy with or without chemotherapy, enrolled in our institution since July 2000. Sixteen patients had glioblastoma multiforme (GBM), six with anaplastic astrocytoma, and three with anaplastic oligodendroglioma. Of the 25 patients, 3 (12%) achieved a complete response (CR), 8 (32%) achieved a partial response (PR), 6 (24%) had stable disease (SD), and 8 (32%) had progressive disease (PD). Two patients achieved a CR, 4 patients achieved a PR, 3 patients had SD and 7 patients had PD in GBM, and 1 patient achieved a CR, 4 patients achieved a PR, 3 patients had SD, 1 patient had PD in the non-GBM patients. Median progression free survival was 8 weeks in GBM and 22 weeks in the non-GBM patients. The median overall survival of each group was 17 weeks and 28 weeks. Temozolomide demonstrated moderate activity in recurrent and progressive malignant gliomas without serious toxicity.
Malignant gliomas, one of the most common primary brain tumors, are very aggressive tumors with poor prognosis despite a multi-modality treatment approach including surgery, radiotherapy and chemotherapy. The median survival time of patients with glioblastoma multiforme (GBM) is 5-12 months from the diagnosis and it increases to 11-36 months for anaplastic astrocytoma (AA) (1). Unfortunately, the standard chemotherapy of nitrosoureas or procarbazine-based regimens has only modest activity and significant toxicity when used in the setting of recurrent malignant gliomas. Therefore, there is considerable interest in new chemotherapy agents such as temozolomide (2).
Temozolomide is a new orally administerable, second-generation imidazotetrazine prodrug with an essentially 100% oral bioavailability. Pre-clinically, temozolomide demonstrates a broad spectrum, schedule dependent, antitumor activity with relatively little toxicity. Temozolomide spontaneously converts to the active alkylating agent 5-(3-methyltriazen-1-yl) imidazole-4-carboximide under physiological conditions with extensive tissue distribution, including a penetration of the blood-brain barrier and into the cerebrospinal fluid (3).
Numerous studies have demonstrated the activity of temozolomide against malignant brain tumors, particularly chemoresistant malignant gliomas (4). Recently, studies have been focusing on the development of strategies to optimize the clinical efficacy of temozolomide by different dosing schedules and combination of other antineoplastic agents. However, no study of temozolomide effect for malignant gliomas has been reported in an Asian population. Here, the authors analyzed a single institution's experience of temozolomide chemotherapy for the patients with recurrent or progressive malignant glioma after surgery and standard radiation therapy with or without chemotherapy.
Eligible patients were over 17 yr old, had a histologically proven malignant glioma (GBM, AA, anaplastic oligodendroglioma) according to the WHO criteria and unequivocal evidence of a tumor recurrence or progression by the any of following imaging modalities: gadolinium magnetic resonance imaging (Gd-MRI); contrast-enhanced computerized tomography (CT) scanning after surgery and standard radiation therapy with or without chemotherapy (at least 4 weeks apart from the completion of previous chemotherapy or radiotherapy). Other eligibility criteria included the following: a Karnofsky performance of at least 60 or more and a life expectancy of more than 12 weeks at the beginning of the temozolomide therapy; adequate hematological, hepatic and renal function; and patient provided written informed consent. The baseline characteristics of all the patients were summarized (Table 1).
Temozolomide was administered orally at a dosage of 150 mg/m2/day (750 mg/m2 total dose per cycle) on day 1 through 5 to the fasting patients who had received previous chemotherapy or at a dosage of 200 mg/m2/day (1,000 mg/m2 total dose per cycle) for patients who had not received previous chemotherapy. The treatment cycles were repeated every 28 days until neurological or radiological deterioration developed. The patients were asked to fast four hours prior to and two hours after administration. A full blood examination was performed prior to each new cycle.
The responses were evaluated according to Macdonald's criteria (5). The first follow-up MRI was performed after 2-3 cycles and the next one after 4-6 cycles. A complete response (CR) was defined as the complete disappearance of all the enhancing or non-enhancing tumors or edema on the MRI (excluding calcification, or MRI T2 periventricular or white matter abnormalities not in continuity with the known tumor mass), with an improvement or stable neurological status without corticosteroid requirements. A partial response (PR) was defined as a greater than 50% decrease in the tumor size, with an improvement or stability in the neurological status. Progressive disease (PD) was defined as a 25% or greater increase in tumor size, the appearance of new lesions, or a progressive neurological decline not explained radiographically and not attributable to any other cause. Stable disease (SD) was defined as a less than 50% regression or less than 25% increase in the tumor size, with stable neurological status and a stable or declining corticosteroid requirement.
Progression-free survival time (PFS) was estimated from the start of temozolomide to tumor progression or to the moment of being withdrawn from the study, and overall survival (OS) from the start of temozolomide to the date of death, irrespective of its cause, or to the last evaluation. PFS and OS were assessed by the product-limit method of Kaplan-Meier for all 25 patients.
From July 2000 to April 2004, 25 patients were evaluable. The median number of treatment cycles was 4 (range 1-15) and the median follow-up period was 15 months (range 2-40 months).
Table 2 summarizes the response rates to treatment. In 16 GBM patients, 2 (12.5%) patient achieved a CR, 4 (25%) patients achieved a PR, and 3 (18.8%) patients had SD. The overall response rate (CR+PR+SD) was 56.3% (Fig. 1). In the 9 non-GBM patients, there were 4 patients with PR (44.4%), 1 patient with a CR (11%) and 3 patients with SD (33.3%). the overall response rate was 88.7% (Fig. 2).
The median progression-free survival of the GBM patients and the non-GBM patients were 8 and 22 weeks, respectively (Fig. 3). The median overall survival of each group was 17 weeks and 28 weeks.
Toxicity grading and dose modification were based on the National Cancer Institute Toxicity Criteria (6). Table 3 summarizes the toxicity data. Nausea and vomiting of grade 1 occurred in 7 patients and was well controlled using standard antiemetics. One patient, who had suffered from the hepatic parenchymal disease before the temozolomide chemotherapy, developed hepatic toxicity of grade 2. Seizures developed in one patient who was on valproic acid. There was no treatment related mortality.
The recurrence of malignant gliomas is associated with significant morbidity and limited survival. Most patients have already received multimodality therapies including surgery, radiotherapy and, on occasion, chemotherapy, at the initial diagnosis. Generally, radiotherapy cannot be administered upon a recurrence, and thus the therapeutic options are limited to a further resection or palliative chemotherapy. Palliative chemotherapy has previously been considered to have a modest efficacy in a recurrent setting. These chemotherapeutic agents do not often result in meaningful clinical improvements and are associated with significant toxicity. In contrast, recent studies have suggested that temozolomide is relatively active in this setting and is well tolerated. Furthermore, the oral formulation of temozolomide is likely to be more acceptable to patients than intravenous regimens.
This study demonstrated significant activity of the drug, with a 68% overall response rate (complete, partial response and stable disease). The notion that stable disease constitutes 'a response' is not generally accepted in the oncology field. However, achieving stable disease in the context of malignant gliomas has been shown to have significant prognostic effects.
This results support other studies using temozolomide in recurrent malignant gliomas. The efficacy of temozolomide in high-grade gliomas has been evaluated in phase II studies in patients at the initial relapse. In a multicenter CRC phase II study, patients with recurrent or progressive high-grade glioma were treated with temozolomide as a first-line therapy. The objective response rate (complete response and partial response) was 11% with a median survival of 5.8 months (7). A similar response rate (8%) and median survival (5.4 months) were observed in a second multicenter phase II trial of temozolomide in a glioblastoma (8). The imaging response rate was 6% and the median survival was 12.4 weeks in a temozolomide arm of a randomized phase II study comparing temozolomide with procarbazine (9). In a phase II study of patients with anaplastic astrocytomas at the initial relapse, the objective response rate to temozolomide was 35%(10). Nevertheless, other studies have suggested that temozolomide improves time to progression and the quality of life but not the overall survival (11-14).
Myelosuppression is the most serious adverse effect of temozolomide, and is dose limiting. However, it does not appear to be cumulative and relatively easily treated. In this study, temozolomide treatment was extremely well tolerated with few significant side effects. Nausea and vomiting were mild and could be well controlled in most cases using standard antiemetics. This study concurs with other studies of temozolomide in which serious side effects were uncommon. In an open label phase II study reported by Brada et al. (8) in glioblastoma multiforme, only 3 out of 132 patients evaluated discontinued therapy because of the adverse events. Grade 3/4 myelosuppression occurred in only 12% of the 414 chemotherapy cycles. Yung et al. (10) reported that only 9 out of 158 anaplastic astrocytoma patients (6%) evaluated discontinued treatment due to the adverse events. Of these patients, 6 discontinued treatment after 6 cycles of therapy. The most frequent adverse events, nausea and vomiting (49%), were readily controlled by standard antiemetics. Other toxicities included fatigue (25%), constipation (23%), and headaches (13%). Interestingly, seizures newly developed in one patient who was on valproic acid even though the drug level was within the therapeutic range and follow-up imaging study showed the decrease in the tumor size with a stablility in the neurological status. It is not clear which factor could be related to the seizure, such as radiation effect or interaction between temozolomide and antiepileptic drugs.
The difficulties in evaluating the response to therapeutic interventions in primary brain tumors are highlighted by this study. The effect of temozolomide in patients who have undergone prior radiotherapy has yet to be established (15). It is plausible that prior radiotherapy should affect the patient's response to temozolomide. In this study, the mean time interval between radiotherapy and temozolomide was 21.5 months (range 2-103 months) and eligible patients developed recurrences even after conventional radiotherapy was completed. It remains unclear how much the later effect of radiotherapy is involved in the response including the temozolomide chemotherapy. Another unresolved issue is the treatment duration. We continued the treatment cycles until the progression or for a maximum of 15 cycles. No cumulative toxic effects or late effects of exposure to an alkylating agent were observed, but the number of patients exposed to long-term therapy is very small. Assessing the activity of new drugs in brain tumors is a difficult task, because measuring of response rates according to the conventional criteria may not reflect the real clinical benefit. New endpoints require a prospective validation and should be supported by a quality-of-life analysis (16,17).
Overall, temozolomide is a new reasonably well tolerable and active oral chemotherapeutic agent for treating malignant gliomas. It has established its place as standard of care for the treatment of recurrent malignant glioma. Future studies may incorporate a combination regimen with other chemotherapeutic agents in both recurrent and adjuvant settings (18). Stupp et al.(19,20) demonstrated that concomitant radiotherapy plus continuous daily temozolomide therapy followed by additional cycles of the standard regimen of adjuvant temozolomide therapy was well tolerated and may prolong the survival in patients with malignant glioma.
Primary temozolomide chemotherapy or chemoradiotherapy for treating metastatic brain tumors is currently under investigation. In addition, the responses to temozolomide have been reported in non-small cell lung cancer, primary central nervous system lymphoma and melanoma (15).
Assessing the true efficacy of temozolomide will require a larger study with a comparison with other agents and treatment modalities.
Figures and Tables
Fig. 1
Pre-chemotherapy MR image (A) and MR image after three courses of temozolomide (B) of a patient with a grade 4 glioma, consistent with a partial response.
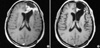
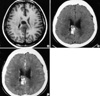
Fig. 2
Pre-chemotherapy MR image (A) showing ill-defined lesion involving the posterior portion of the corpus callosum of a patient with a grade 3 glioma. Non-enhanced CT scan (B) and enhanced CT scan (C) after fourteen cycles of temozolomide, consistent with a complete response.
Notes
References
1. Burger PC, Vogel FS, Green SB, Strike TA. Glioblastoma multiforme and anaplastic astrocytoma. Cancer. 1985. 56:1106–1111.
2. O'Reilly SM, Newlands ES, Glaser MG, Brampton M, Rice-Edwards JM, Illingworth RD. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours. Eur J Cancer. 1993. 29:940–942.
3. Stevens MF, Hickman JA, Langdon SP, Chubb D, Vickers L, Stone R. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987. 47:5846–5852.
4. DeAngelis LM. Chemotherapy for brain tumors. N Engl J Med. 2005. 352:1036–1038.
5. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990. 8:1277–1280.

6. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981. 47:207–214.

7. Bower M, Newlands ES, Bleehen NM, Brada M, Begent RJ, Calvert H, Colquhoun I, Lewis P, Brampton MH. Multicentre CRC phase II trial of temozolomide in recurrent or progressive high-grade glioma. Cancer Chemother Pharmacol. 1997. 40:484–488.

8. Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, Stupp R, Yue N, Bruner J, Dugan M, Rao S, Zaknoen S. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol. 2001. 12:259–266.

9. Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000. 83:588–593.

10. Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O'Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. J Clin Oncol. 1999. 17:2762–2771.

11. Brandes AA, Ermani M, Basso U, Amistà P, Berti F, Rotilio A, Pinna G, Gardiman M, Monfardini S. Temozolomide as a second-line systemic regimen in recurrent high-grade glioma: a phase II study. Ann Oncol. 2001. 12:255–257.

12. Dinnes J, Cave C, Huang S, Milne R. A rapid and systemic review of the effectiveness of temozolomide for the treatment of recurrent malignant glioma. Br J Cancer. 2002. 86:501–505.
13. Gaya A, Rees J, Greenstein A, Stebbing J. The use of temozolomide in recurrent malignant gliomas. Cancer Treat Rev. 2002. 28:115–120.

14. Harris MT, Rosenthal MA, Ashley DL, Cher L. An Australian experience with temozolomide for the treatment of recurrent high grade gliomas. J Clin Neurosci. 2001. 8:325–327.

15. Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumors. Lancet Oncol. 2001. 2:552–560.
16. Groenvold M, Klee MC, Sprangers MA, Aaronson NK. Validation of the EORTC QLQ-C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol. 1997. 50:441–450.

17. Osoba D, Aaronson NK, Muller M, Sneeuw K, Hsu MA, Yung WKA, Brada M, Newlands E. Effect of neurological dysfunction on healthrelated quality of life in patients with high-grade glioma. J Neurooncol. 1997. 34:263–278.
18. Groves MD, Puduvalli VK, Hess KR, Jaeckle KA, Peterson P, Yung WKA, Levin VA. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol. 2002. 20:1383–1388.

19. Stupp R, Dietrich P-Y, Kraljevic SO, Pica A, Maillard I, Maeder P, Meuli R, Janzer R, Pizzolato G, Miralbell R, Porchet F, Regli L, Tribolet N, Mirimanoff RO, Leyvraz S. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002. 20:1375–1382.

20. Stupp R, Mason WP, Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352:987–996.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download