Abstract
Background and Objectives
Although increasing evidence has indicated that radial access is a beneficial technique, few studies have focused on Korean subjects. The aim of this study was to evaluate current practice of coronary angiography (CAG) and percutaneous coronary intervention (PCI) using radial access in South Korea.
Subjects and Methods
A total of 6338 subjects were analyzed from Korean Transradial Intervention prospective registry that was conducted at 20 centers in Korea. After evaluating the initial access, subjects intended for radial access were assessed for their baseline, procedure-related, and complication data. Subjects were categorized into three groups: group of overall subjects (n=5554); group of subjects who underwent PCI (n=1780); and group of subjects who underwent primary percutaneous coronary intervention (PPCI) (n=167).
Results
The rate of radial artery as an initial access and the rate of access site crossover was 87.6% and 4.4%, respectively, in overall subjects. Those rates were 82.4% and 8.1%, respectively, in subjects who underwent PCI, and 60.1% and 4.8%, respectively, in subjects who underwent PPCI. For subjects who underwent CAG, a 6-F introducer sheath and a 5-F angiographic catheter was the most commonly used. During PCI, a 6-F introducer sheath (90.6%) and a 6-F guiding catheter were standardly used.
The application of radial access to coronary angiography (CAG) and percutaneous coronary intervention (PCI) has been steadily growing worldwide. This increased application is partly due to findings that radial access can reduce the rate of access site complications, thereby reducing patient mortality. This trend is especially true for patients with ST-segment elevation myocardial infarction (STEMI).1) Currently, American and European guidelines recommend the use of radial access during PCI.2)3) However, this technique is associated with a learning curve, which can increase the time required to perform the procedure. Thus, the use of radial access is still limited.4) Although increasing evidence has suggested that radial access is a beneficial technique, few published articles have focused on Korean subjects. Members of the Korean Transradial Intervention (KOTRI) working group has prospectively collected data from subjects who underwent CAG or PCI. Therefore, the objective of this study was to use the data from the KOTRI registry and evaluate the current practice of CAG and PCI using radial access.
The KOTRI prospective registry was conducted at 20 centers (122 operators) in Korea. Subjects who underwent CAG and PCI for 6 months (February 2014 to July 2014) were enrolled in the registry regardless of the access site. Due to delays in obtaining Institutional Review Board (IRB) approvals, the study period was extended for some centers. A total of 6793 subjects were initially enrolled in this registry. However, subjects less than 18 years of age, subjects whose case report forms (CRF) lacked over 20% of the data, and subjects with inappropriate data were excluded. Finally, a total of 6338 subjects were included in this analysis (Fig. 1). To reflect the real-world practice, all subjects except those who did not agree to participate in this registry were enrolled.
Two types of CRF (brief form and full form) were entered into the KOTRI prospective registry. The brief form included procedural data as a summary. Some variables of echocardiography, radial angiographic findings, and laboratory findings were omitted from the brief form. Of the 20 centers, 9 centers with 2398 subjects used the brief form while 11 centers with 3945 subjects used the full form.
All subjects were followed clinically for one month to evaluate the patency of the access site. Subjects who underwent PCI were followed for one year to evaluate major adverse cardiac event. This study was approved by each local IRB. All subjects provided written informed consent. This trial was registered at the National Institutes of Health Clinical Trials Registry (ClinicalTrials.gov identifier: NCT01803841).
A comprehensive description of all KOTRI prospective registry data elements and definitions are available at http://tricrf.medsoft.co.kr. All data elements and definitions were prospectively defined by a committee selected from the KOTRI working group. Access site crossover was defined as a change in the access site between the start of the procedure and the end of the procedure. Radial failure was defined as a change of the access site to a non-radial artery. Bleeding was defined according to a consensus report from Bleeding Academic Research Consortium (BARC).5) Puncture time was the duration (min) between lidocaine infiltration and the completion of sheath insertion. CAG time (min) was the duration between the completion of sheath insertion and the end of CAG. PCI time (min) was the duration between the end of CAG and the end of PCI. Total procedure time was the sum of the puncture, CAG, and PCI times. Total contrast volume and total fluoroscopy time were the sums of appropriate parts.
Continuous variables were expressed as mean±standard deviation or median values with interquartile ranges. Categorical variables were expressed as number (percentage). All statistical analyses were performed using SPSS-PASW software, version 20.0 (IBM, Armonk, NY, USA). Statistical significance was considered when a p was less than 0.05.
Initial access site is presented in Fig. 2. In overall subjects, the rate of radial access was 87.6% (right radial: 70.5%; left radial: 29.5%) and the rate of femoral access was 12.3% (right femoral: 92.9%; left femoral: 7.1%). However, the rate of radial access was decreased to 82.4% in subjects who underwent PCI and 60.1% in subjects who underwent primary percutaneous coronary intervention (PPCI).
The baseline characteristics of subjects intended for radial access are presented in Table 1. For overall subjects, their mean age was 64 years and 61% of them were males. The rates of hypertension and diabetes were 46% and 21%, respectively. Non-significant coronary artery disease was present in 51% of the overall subjects. For subjects who underwent PCI, their mean age was 66 years and 51% of them were males. The rate of comorbidity in the group of subjects who underwent PCI was higher than that of the group of overall subjects. The incidence of acute myocardial infarction was 25% in subjects who underwent PCI.
Rates of access site crossover for subjects intended for radial access are summarized in Table 2 and Fig. 3. The rate of access site crossover was 4.4% in the group of overall subjects. It was increased to 8.1% in the group of subjects who underwent PCI. However, the rate of access site crossover was decreased to 4.8% in the group of subjects who underwent PPCI. The most common direction of access site crossover was a crossover from radial artery to femoral artery in all three groups of subjects. The most common cause of access site crossover in the group of overall subjects was puncture failure (35.2%), followed by vessel tortuosity (16.4%). In contrast, the most common cause of access site crossover in the group of subjects who underwent PCI was routine practice (20.1%), followed by vessel tortuosity (19.4%) and the requirement of a larger catheter (18.8%). Routine practice was defined as when radial access was used during CAG but femoral access was used for PCI.
Rates of bleeding and access site complications in all subjects intended for radial access are shown in Table 3. The rate of bleeding complication was 1.2% for the group of overall subjects, 1.9% for the group of subjects who underwent PCI, and 4.8% for the group of subjects who underwent PPCI. Although type 1 bleeding was the most common one in all groups, the rates of type 2 and type 3a bleeding were increased in the group of subjects who underwent PCI and those who underwent PPCI. The rate of access site complication was 0.8% in the group of overall subjects and 1.6% in the group of subjects who underwent PCI. The most common type of access site complication was minor hematoma.
The sizes of the introducer sheath and catheter used in subjects who underwent procedures using transradial access are shown in Fig. 4. Of subjects who underwent CAG, a 6-F introducer sheath was the most commonly used (43.9%), followed by a 5-F introducer sheath (37.6%). However, a 5-F angiographic catheter was the most commonly used one during CAG. During PCI, a 6-F introducer sheath (90.6%) and a 6-F guiding catheter (89.7% for right coronary artery; 90.4% for left coronary artery) were the most commonly used. The rate of ≥ 2 of diagnostic catheters used was 2.1% during right coronary angiography and 2.0% during left coronary angiography. The rate of ≥ 2 of guiding catheters used was 8.1% during right coronary intervention and 6.4% during left coronary intervention.
Procedure time, contrast volume, and fluoroscopy time in subjects who underwent procedures using radial access (after excluding subjects with access site crossover) are shown in Fig. 5. The median puncture time was 2 min in subjects who underwent CAG and in subjects who underwent PCI. However, the median puncture time was decreased to 1 min in subjects who underwent PPCI. The median CAG time was 9 min in subjects who underwent CAG and 8 min in subjects who underwent PCI. The median CAG time was also decreased to 5 min in subjects who underwent PPCI. The median PCI time in subjects who underwent PCI was 25 min. The median contrast volume was 70 mL during CAG and 180 mL during CAG plus ad-hoc PCI. The median fluoroscopy time was 3 min during CAG and 11 min during CAG plus ad-hoc PCI.
In this study, we presented an overview of various data from subjects who underwent CAG and/or PCI via radial access. Data presented here included rates of access site crossover, rates of bleeding, rates of access site complications, selections of introducer sheath and catheter, procedure times, contrast volumes, and fluoroscopy times for various subgroups of subjects. Data were collected from the KOTRI prospective registry, a large multicenter prospective registry containing data from 6338 subjects at 20 centers in Korea. In this registry, the radial artery was used as an initial access site in 87.6% of all subjects. However, the use of radial access was decreased during PCI (82.4%) and further decreased during PPCI (60.1%).
The rate of access site crossover was 4.4% in overall subjects. This rate was increased to 8.1% in the group of subjects who underwent PCI. However, this rate was decreased to 4.8% in the group of subjects who underwent PPCI. One early phase trial found that the rate of access site crossover during radial access was relatively high (7.3%).6) However, other studies employing more advanced devices and techniques have reported a much lower crossover rate (1.5%).7)8) In this registry, the rate of access site crossover was 4.4% in overall subjects. This rate is similar to that seen at a single center with a high volume of radial procedures (4.9%) and the highest tertile of radial access PCI volumes reported in the RadIal Vs femorAL access for coronary intervention (RIVAL) trial (4.4%).9)10) Since not all centers and operators in our registry were specialized in radial procedures, the rate of access site crossover was increased to 8.1% in subjects who underwent PCI. This value is similar to that of the intermediate tertile (9.7%) and the lowest tertile (8.0%) of radial access PCI volumes from the RIVAL trial.10) Interestingly, this rate was decreased to 4.8% in subjects who underwent PPCI. We hypothesize that PCI using radial access is most effective for patients with STEMI when it is performed by centers with high volumes of radial procedure or operators with extensive experience in radial access. However, further analysis is needed to thoroughly test this hypothesis.
In this registry, the most common reason for radial access crossover in overall subjects was puncture failure (35.2%), followed by vessel tortuosity (16.4%). This result is similar to those reported in previous studies.11)12) However, the two common reasons for radial access crossover in subjects underwent PCI were routine practice (20.1%) and the requirement of larger catheter (18.8%). There are concerns about radial artery spasm and occlusion during PCI for patients with small radial artery. Although a study in Japan has reported that the average radial artery size is smaller than the outer diameter of a 6-F introducer sheath in 14.3% of all males and 27.4% of all females,13) the rate of radial occlusion can vary from 1.5% to 33%. Moreover, up to 50% of all radial occlusions can resolve spontaneously within one month.14)15)16)17) The risk of radial artery occlusion can be mitigated through actions such as proper compression, use of sheathless or small guiding catheters, and proper anticoagulation. Considering the complexity of the procedure, most cases involving bifurcation PCI (except simultaneous use of two stents), rotablator use (up to a 1.75 mm burr), thrombus aspiration, and intravascular ultrasound (IVUS) can be safely performed using a 6-F guiding catheter. Therefore, a 6-F guiding catheter can be safely used in most cases. In fact, most PCI cases in our registry were performed with a 6-F guiding catheter. Accordingly, a routine practice or the requirement of a larger catheter for access site crossover would be decreased as operator's experience in radial access increases.
The most important benefit of radial access is that this technique can reduce the chance of access site-related bleeding compared to a femoral access approach. This benefit has been confirmed by meta-analysis of small randomized trials, large randomized trial (the RIVAL trial), and large observational registries.10)18)19)20)21) In this registry, the rate of bleeding was 1.2% in overall subjects, 1.9% of subjects who underwent PCI, and 4.8% of subjects who underwent PPCI. Since most bleeding was classified as BARC type 1 or 2, clinically significant bleeding was only 0.2% in overall subjects, 0.3% in subjects who underwent PCI, and 1.2% in subjects who underwent PPCI. The rate of clinically significant bleeding (1.2%) in subjects who underwent PPCI is lower than that of Acute Catheterization and Urgent Intervention Triage Strategy major bleeding (1.9%) reported in the RIVAL trial.
Since access site complications were reported in two subjects who underwent PPCI and that both of these complications were related to minor hematomas, we infer that non-access site major bleedings were involved in these cases. One pooled analysis suggested that non-access site major bleeding has a stronger effect on prognosis compared to access site major bleeding.22) Thus, reducing both access-related and non-access site-related bleeding by using radial access and optimized anticoagulation approaches should be considered.
Although many studies have suggested that vascular access site complications and site-related instances of major bleeding can be reduced by using radial access,10)23) radial access is not completely free of vascular complications. In this registry, the rate of major vascular complications including dissection, major hematoma, perforation, pseudoaneurysm, hematoma requiring transfusion, and arteriovenous fistula was 0.3% in overall subjects. Vascular complications are influenced by a number of factors, including operator experience, vascular size, vascular tortuosity, and hemostatic method. Thus, operator technique, patient selection, and vascular complication monitoring by the cathlab team must be optimal to minimize access site-related complications.
In this registry, the rate of radial artery occlusion was 0%. Although radial access did not lead to any immediate complications, true incidence of radial artery occlusion may have been underestimated due to its asymptomatic nature.
As mentioned earlier, most PCI could be performed with a 6-F guiding catheter. However, 5-F guiding catheter has been shown to minimize radial and coronary artery injury.24)25) In this registry, the most commonly used guiding catheter size was 6-F. However, some centers showed preference for 5-F guiding catheters (Fig. 4). Previous studies reported lower rates of radial occlusion in small guiding catheter (4-F or 5-F) groups compared to 6-F groups. However, due to small sample sizes, these differences were not significant.25)26) Thus, whether guiding catheter size has any effect on radial complications such as radial artery occlusion needs to be determined in future studies.
During coronary angiography, the most commonly used angiographic catheter size was 5-F, followed by 4-F However. Although 4-F and 5-F angiographic catheters were the most commonly used, the most prevalent introducer sheath size was 6-F. This discrepancy may be due to cost issue considering the fact that PCI using 6-F is performed ad-hoc in some centers.
Compared to femoral access, there are two concerns associated with radial access: 1) This technique is associated with increased procedure time; 2) It is associated with increased radiation exposure compared to femoral access.10)27) These concerns are especially relevant for patients with STEMI. Radial access might increase door-to-balloon time. Thus, it might be associated with poor clinical outcome.28)
In this registry, the median puncture time was 2 min and the median CAG time was 9 min during CAG (with or without ad-hoc PCI). However, the median puncture time was decreased to 1 min and the median CAG time was decreased to 5 min in subjects who underwent PPCI. Considering the non-random nature of this registry, it is likely that subjects who underwent PPCI were enrolled at experienced centers with expert operators.
Compared to the RIVAL trial, the median contrast volume of subjects who underwent PPCI was smaller in the present registry (181 mL vs. 170 mL, respectively). However, the median total procedure time (35 min vs. 37 min, respectively) and fluoroscopy time (9.3 min vs. 11.0 min, respectively) were longer in the present registry.10) Heterogeneity in procedure and fluoroscopy times among centers can probably be explained by differences in angulated view takes, procedure methods such as the use of pressure wire, and the use of IVUS by centers and operators.
This study has several limitations. First, most participating centers are interested in radial access or have experience in radial approaches (>80% radial access in 14 centers). Thus, results from this registry may not completely reflect the current practices in South Korea. Second, although we planned to enroll all subjects who underwent CAG or PCI regardless of access site for 6 months, some centers did not enroll all subjects. Thus, selection bias might have been introduced. However, the rate of radial access based on results of survey from 12 active participation centers (8574 subjects) was similar to that of this current registry: 82.8% during CAG, 72.0% during PCI, and 63.1% during PPCI. Third, since each center enrolled a different number of subjects, centers that enrolled large numbers of subjects may have disproportionately influenced the study results. Finally, although a number of subjects were excluded due to the lack of data, some missing data still exist in this registry.
Figures and Tables
Fig. 1
Data set for the KOTRI prospective registry and initial access site for coronary angiography or intervention. CAG: coronary angiography, PCI: percutaneous coronary intervention, KOTRI: Korean transradial intervention, PPCI; primary percutaneous coronary intervention.
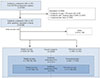
Fig. 2
Initial access site. Institution codes presented as capitalized letter A to T are listed in the Supplementary Table (in the online-only Data Supplement). PCI: percutaneous coronary intervention, PPCI: primary percutaneous coronary intervention, TF: transfemoral, TR: transradial.

Fig. 3
Access site crossover in subjects intended for transradial access. Institution codes presented as capitalized letter A to T are listed in the Supplementary Table (in the online-only Data Supplement). PCI: percutaneous coronary intervention, PPCI: primary percutaneous coronary intervention.

Fig. 4
Introducer sheath and catheter size in subjects who underwent procedures using radial access (after excluding subjects with access site crossovers). Institution codes presented as capitalized letter A to T are listed in the Supplementary Table (in the online-only Data Supplement). CAG: coronary angiography, PCI: percutaneous coronary intervention.

Fig. 5
Procedure time and contrast volume in subjects who underwent procedures using radial access (after excluding subjects with access site crossover). Institution codes presented as capitalized letter A to S (T) are listed in the Supplementary Table (in the online-only Data Supplement). CAG: coronary angiography, PCI: percutaneous coronary intervention, PPCI: primary percutaneous coronary intervention.

Table 1
Baseline characteristics of subjects intended for radial access

Data are mean±standard deviation or n (%). PCI: percutaneous coronary intervention, PPCI: primary percutaneous coronary intervention, CKD: chronic kidney disease, MI: myocardial infarction, CABG: coronary artery bypass graft, CVA: cerebrovascular attack, NSTEMI: non-ST-segment elevation myocardial infarction, STEMI: ST-segment elevation myocardial infarction, CAD: coronary artery disease, VD: vessel disease
Table 2
Access site crossover in subjects intended for radial access

Table 3
Bleeding and access site complications in subjects intended for radial access

Acknowledgments
The KOTRI prospective registry was supported by a grant (2012-1) from the Korean Society of Interventional Cardiology. The Korean Society of Interventional Cardiology was not involved in the study design, data collection, data analysis, or writing of this report. The authors of this manuscript and the steering committee are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
KOTRI prospective registry investigators and sites: Junghan Yoon (principal investigator), Yonsei University Wonju Severance Christian Hospital, Wonju, Korea (1098 subjects enrolled); Seung-Woon Rha from Korea University Guro Hospital, Seoul, Korea (817); Kyung-Soo Kim from Hanyang University Seoul Hospital, Seoul, Korea (620); Jae-Hwan Lee from Chungnam National University Hospital, Daejeon, Korea (491); Myung Ho Jeong from Chonnam National University Hospital, Gwangju, Korea (475); Yun-Hyeong Cho from Kwandong Univeristy Myongi Hospital, Goyang, Korea (448); Keum-Soo Park from Inha University Hospital, Incheon, Korea (282); Kwang Soo Cha from Pusan University Hospital, Busan, Korea (258); Dae-Hee Shin, from Ulsan University Gangneung Asan Hospital, Gangneung, Korea (243); Jin-Bae Lee from Daegu Catholic University Medical Center, Daegu, Korea (242); Jang-Ho Bae from Konyang University Hospital, Daejon, Korea (220); Kyoo-Rok Han, from Hallym University Kandgong Sacred Heart Hospital, Seoul, Korea (209); Doo-Il Kim from Inje University Haeundae Paik Hospital, Busan, Korea (208); Byung-Ryul Cho from Kangwon National University Hospital, Chuncheon, Korea (201); Sang Wook Kim from Chung-Ang University Hospital, Seoul, Korea (188); Hee-Yeol Kim from Catholic University Bucheon St. Mary's Hospital, Bucheon, Korea (90); Jon Suh from Soon Chun Hyang University Bucheon Hospital, Bucheon, Korea (86); Sung-Ho Her from Catholic University Daejeon St. Mary's Hospital Daejeon, Korea (79); Min Su Hyon from Soon Chun, Hyang University Seoul Hospital, Seoul, Korea (72); and Jae Woong Choi from Eulji University Medical Center, Daejeon, Korea (18).
References
1. Mamas MA, Ratib K, Routledge H, et al. Influence of access site selection on PCI-related adverse events in patients with STEMI: meta-analysis of randomised controlled trials. Heart. 2012; 98:303–311.
2. Authors/Task Force members. Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014; 35:2541–2619.
3. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011; 58:e44–e122.
4. Ball WT, Sharieff W, Jolly SS, et al. Characterization of operator learning curve for transradial coronary interventions. Circ Cardiovasc Interv. 2011; 4:336–341.
5. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011; 123:2736–2747.
6. Agostoni P, Biondi-Zoccai GG, de Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004; 44:349–356.
7. Pristipino C, Pelliccia F, Granatelli A, et al. Comparison of access-related bleeding complications in women versus men undergoing percutaneous coronary catheterization using the radial versus femoral artery. Am J Cardiol. 2007; 99:1216–1221.
8. Vink MA, Amoroso G, Dirksen MT, et al. Routine use of the transradial approach in primary percutaneous coronary intervention: procedural aspects and outcomes in 2209 patients treated in a single high-volume centre. Heart. 2011; 97:1938–1942.
9. Burzotta F, Trani C, Mazzari MA, et al. Vascular complications and access crossover in 10,676 transradial percutaneous coronary procedures. Am Heart J. 2012; 163:230–238.
10. Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011; 377:1409–1420.
11. Pristipino C, Roncella A, Trani C, et al. Identifying factors that predict the choice and success rate of radial artery catheterisation in contemporary real world cardiology practice: a sub-analysis of the PREVAIL study data. EuroIntervention. 2010; 6:240–246.
12. Dehghani P, Mohammad A, Bajaj R, et al. Mechanism and predictors of failed transradial approach for percutaneous coronary interventions. JACC Cardiovasc Interv. 2009; 2:1057–1064.
13. Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv. 1999; 46:173–178.
14. Spaulding C, Lefèvre T, Funck F, et al. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn. 1996; 39:365–370.
15. Stella PR, Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet Cardiovasc Diagn. 1997; 40:156–158.
16. Sanmartin M, Gomez M, Rumoroso JR, et al. Interruption of blood flow during compression and radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv. 2007; 70:185–189.
17. Steffenino G, Fabrizi Mde B, Baralis G, et al. Implementation of radial arterial access for cardiac interventions: a strong case for quality assurance protocols by the nursing staff. J Cardiovasc Med (Hagerstown). 2011; 12:116–121.
18. Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009; 157:132–140.
19. Montalescot G, Ongen Z, Guindy R, et al. Predictors of outcome in patients undergoing PCI. Results of the RIVIERA study. Int J Cardiol. 2008; 129:379–387.
20. Chase AJ, Fretz EB, Warburton WP, et al. Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg). Heart. 2008; 94:1019–1025.
21. Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008; 1:379–386.
22. Verheugt FW, Steinhubl SR, Hamon M, et al. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011; 4:191–197.
23. Karrowni W, Vyas A, Giacomino B, et al. Radial versus femoral access for primary percutaneous interventions in ST-segment elevation myocardial infarction patients: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2013; 6:814–823.
24. Hamon M, Pristipino C, Di Mario C, et al. Consensus document on the radial approach in percutaneous cardiovascular interventions: position paper by the European Association of Percutaneous Cardiovascular Interventions and Working Groups on Acute Cardiac Care** and Thrombosis of the European Society of Cardiology. EuroIntervention. 2013; 8:1242–1251.
25. Dahm JB, Vogelgesang D, Hummel A, Staudt A, Völzke H, Felix SB. A randomized trial of 5 vs. 6 French transradial percutaneous coronary interventions. Catheter Cardiovasc Interv. 2002; 57:172–176.
26. Takeshita S, Asano H, Hata T, et al. Comparison of frequency of radial artery occlusion after 4Fr versus 6Fr transradial coronary intervention (from the Novel Angioplasty USIng Coronary Accessor Trial). Am J Cardiol. 2014; 113:1986–1989.
27. Mercuri M, Mehta S, Xie C, Valettas N, Velianou JL, Natarajan MK. Radial artery access as a predictor of increased radiation exposure during a diagnostic cardiac catheterization procedure. JACC Cardiovasc Interv. 2011; 4:347–352.
28. Baklanov DV, Kaltenbach LA, Marso SP, et al. The prevalence and outcomes of transradial percutaneous coronary intervention for ST-segment elevation myocardial infarction: analysis from the National Cardiovascular Data Registry (2007 to 2011). J Am Coll Cardiol. 2013; 61:420–426.
Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.4070/kcj.2015.45.6.457.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download