Abstract
We reported a case of a 55-year-old patient who presented with palpitation after swallowing. Initial surface electrocardiogram revealed ventricular preexcitation utilizing a left lateral bypass tract. The orthodromic atrioventricular reentrant tachycardia (AVRT) was induced during electrophysiologic studies. After successful ablation of the AVRT utilizing a left lateral free wall bypass tract, 2 different atrial tachycardias (ATs) were induced under isoproterenol infusion. When the patient swallowed saliva or drank water, 2 consecutive beats of atrial premature complexes (APCs) preceded another non-sustained AT repeatedly, which was coincident with the patient's symptom. The preceding APC couplet had the same activation sequence with one induced AT, and the subsequent non-sustained AT had the same activation sequence with the other induced AT, respectively. We first targeted the preceding 2 consecutive APCs at the left posterior interatrial septum. The following non-sustained AT was also eliminated following ablation of the APCs. After ablation, the patient remained free from the swallowing-induced atrial tachyarrhythmias during the one year follow-up.
Swallowing-induced atrial tachyarrhythmia is a rare clinical phenomenon. Recently a case was reportedly cured by radiofrequency catheter ablation (RFCA).1)2)3)4) We reported 2 different types of atrial tachycardia (AT) as a similar activation sequence of 2 consecutive beats of atrial premature complexes (APCs) followed by non-sustained AT, which were reproducibly triggered by swallowing or dr-inking water. They were successfully ablated at the left posterior interatrial septum at the time of targeting 2 preceding beats of APCs in a patient with Wolff-Parkinson-White (WPW) syndrome, utilizing a left lateral bypass tract which was incidentally accompanied by these rare occurrences.
A 55-year-old man suffered from recurrent palpitation after swallowing saliva or drinking water. A baseline surface electrocardiogram (ECG) revealed ventricular preexcitation utilizing a left lateral bypass tract (Fig. 1A). During palpitation, ECG revealed 2 different morphologies of supraventricular tachycardia. The first one was a narrow, regular QRS tachycardia with a heart rate of 240 bpm, suggesting atrioventricular (AV) reenrant tachycardia (Fig. 1B) and the second one was a short episode of an irregular atrial tachyarrhythmias with rapid ventricular response related to preexcitation lasting for 7 beats, which was composed of 2 consecutive APCs followed by non-sustained AT (Fig. 1C). The patient was referred to our clinic for RFCA for recurrent episodes of tachycardia refractory to calcium channel blockers. He had a 7-year history of diabetes. Two-dimensional echocardiography demonstrated normal cardiac structure with preserved ventricular function. After obtaining written inform-ed consent, an electrophysiologic study was performed under local anesthesia. A multielectrode duodecapolar catheter (St. Jude Medical, St. Paul, MN, USA) was placed in the coronary sinus (CS) via the right femoral vein, and 2 quadripolar catheters were placed in the His bundle region and right ventricular apex via the left femoral vein. Preexcitation was shown at baseline (Fig. 2A). Basic interval was measured. Atria-His (AH) interval was 68 ms and His-ventricular (HV) interval was 35 ms. The Atrioventricular (AV) block cycle length was 280 ms. Two different activation sequences of APCs with non-sustained AT were intermittently present. The earliest atrial activation of the preceding APCs was near the CS ostium (CS 9,10 area), and that of the following non-sustained AT was near the inferior-lateral wall of the right atrium (RA 5, 6 area). Programmed electrical stimulation was performed in an attempt to induce clinical tachycardia. Initially, orthodromic AV reentrant tachycardia was induced by double ventricular extrastimuli, with retrograde conduction by the left lateral free wall bypass tract (Fig. 2B). A 7 Fr 4-mm tip ablation catheter (Boston Scientific, Boston, MA, USA) was introduced into the left atrium (LA) through a transseptal puncture, and radiofrequency (RF) energy application was successfully performed to ablate the left lateral bypass tract (Fig. 2C). However, following ablation, atrial burst pacing 250 ms under isoproterenol (3 µg/min) induced 2 different types of sustained ATs (AT1 and AT2). And AT2 was also initiated spontaneously (Fig. 3A). The 2 different inducible ATs demonstrated the same atrial activation sequence with previously recorded APCs and non-sustained AT, respectively (Fig. 3B). The patient was asked to swallow cold water while the catheters were placed; although it was difficult to induce APCs and AT by pacing stimulation during isoproterenol infusion, swallowing reproducibly induced 2 consecutive APC beats followed by different activation sequences of short runs of non-sustained AT. Their atrial activation sequence was earlier in the CS ostium, followed by the inferior-lateral wall of the tricuspid annulus, which was recorded by the CS multielectrode catheter (Fig. 3B). During mapping at the left posterior interatrial septum to target the preceding APC couplet rather than the following non-sustained AT, the earliest site of activation was found to be 25 ms before the onset of the surface P wave (Fig. 3C). Nonirrigated RF current with temperature-controlled mode was delivered to this location, with a target temperature of 60℃ and a maximum power output of 50 W for 90 seconds. The APCs and non-sustained AT were completely abolished. Total procedure time and fluoroscopic time were 170 and 45 minutes, respectively. Following RF ablation, attempts to induce tachycardia, including programmed electrical and burst pacing from the RA and RV, were undertaken. No AV reenrant tachycardia, nor AT was induced, and repeated swallowing failed to reproduce any APC or non-sustained AT. A gastrointestinal workup, including an upper gastrointestinal endoscopy, was unremarkable. The patient has been free from swallowing-induced atrial arrhythmias during the one year of follow-up.
Swallowing-induced tachyarrhythmia is rare and the prevalence is reported as 0.6% among patients who underwent RFCA for atrial arrhythmias.1) APCs and/or AT are the most common presenting arrhythmias. Moreover, AV nodal reentrant tachycardia5) and AV reentrant tachycardia6) as well as atrial tachyarrhythmias including atrial fibrillation, can be induced by swallowing.7) We presented a similar case in the present report of a patient with WPW syndrome. We initially performed ablation of the left lateral free wall bypass tract, which did not seem to be mediated by swallowing. Thereafter, 2 different morphologies of sustained ATs were induced by atrial burst pacing under isoproterenol infusion. The morphology of initially induced AT was suspected to be near the CS ostium origin, and the subsequent AT was suspected to originate from the inferior-lateral wall of the tricuspid annulus origin. Coincidently, 2 consecutive APCs, followed by different morphologies of non-sustained AT, reproducibly occurred after swallowing or drinking water. The 2 different types of induced ATs were subsequently eliminated by catheter ablation, targeting the preceding 2 consecutive APCs beats at a time. Therefore, we believe that 2 different inducible ATs might be the fundamental arrhythmias of the swallowing-mediated atrial tachyarrhythmias. Prior studies have shown that most patients have no evidence of structural heart or esophageal disease.1)2)3) The mechanism of these arrhythmias is unclear, but a vagally-mediated neural reflex during episodes of swallowing is accepted as a potential mechanism.1) Some authors have previously suggested that there is direct mechanical stimulation of the LA by a distended esophagus.2) However, direct mechanical interaction between the distended esophagus and adjacent LA is unlikely in the present case because the origin of the tachycardia was the left posterior interatrial septum, which is far from the esophagus, as identified by barium swallowing. Another proposed mechanism is that of sympathetic reflexes originating in the esophagus during swallowing.1)4) On the basis of the present case and previously reported cases, it is speculated that the majority of these atrial tachyarrhythmias arise from an autonomous ectopic focus. Additionally, the relationship between swallowing-induced ATs and WPW syndrome utilizing a left lateral bypass tract is not clear. It seemed that WPW syndrome was incidentally accompanied by these rare occurrences. Yamauchi et al.8) first demonstrated that curative catheter ablation therapy of this arrhythmia was performed by extensive right superior pulmonary vein (PV) antral isolation. Recently, another study also demonstrated swallowing-induced multifocal AT originating from the right PV, which was abolished by extensive isolation of ipsilateral right-sided PVs.9) There are only a few case reports regarding ablation therapy in this situation.1)8)9)10)11) We performed mapping of atrial arrhythmia during swallowing or drinking water, in the present case. This aided the ablation therapy as well as the feasibility of mapping during the procedure in a conscious patient. To date, several patients underwent RFCA using focal or extensive PV antral ablation. Most patients demonstrated PV tachycardia. However, a non-PV foci of AT was reported in 2 cases in the low posterior RA area. The successful ablation site of 2 different atrial tachyarrhythmias, the first of which might have been related with the following one, was the left posterior interatrial septum near the right inferior PV. However, the exact abolishment site of the identified ATs could not be confirmed because we unfortunately did not use the 3-dimensional electroanatomic mapping system intra-procedurally. Although we performed point by point activation mapping in the near right inferior PV ostium, left anterior interatrial septum, and left atrial posterior wall, as well as left posterior interatrial septum under the fluoroscopic guidance, there was still some possibility of ostitum of the right inferior PV near the right inferior ganglionated plexus (GP) area related to the ablation site. The precise mechanism of how targeting the APCs could eliminate other following nonsustained AT is not clear. Recently, epicardial adipose tissue (EAT) was shown to be related to the mechanism of swallowing-induced AT as a neural reflex.11) It was found that EAT was adjacent to the endocardial breakthrough of this rare type of AT. EAT contains autonomic GP, such as both adrenergic and cholinergic nerves. These intrinsic cardiac ganglia interact with the extrinsic autonomic nerve system to modulate cardiac electrophysiology.12) Simultaneous activation of these nerve structures in response to the extrinsic cardiac stimulation by autonomic nerve system may enhance triggered activity and facilitate the development of these 2 different type of ATs. Therefore, EAT could be considered as a mechanism of AT in this clinical setting. We speculated that the mechanism of single spot abolishment of 2 different ATs may be related to the extrinsic connection of EAT between one triggering site and the other, although we did not fully evaluate it by the cardiac computed tomography or magnetic resonance imaging.
Figures and Tables
Fig. 1
Baseline 12-lead surface electrocardiogram and clinically documented dual morphology supraventricular tachycardias. A: the delta wave morphology of the Wolff-Parkinson-White syndrome suggests the left lateral free wall bypass tract. B: the narrow regular QRS tachycardia, suggesting atrioventricular reentrant tachycardia (AVRT). C: the short run of irregular atrial tachyarrhythmias with rapid ventricular response related to preexcitation, composed of 2 different types of atrial premature complexes (*) and non-sustained atrial tachycardia (AT, arrows).
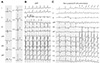
Fig. 2
Wolff-Parkinson-White syndrome and successful ablation site of bypass tract. A: the intra-cardiac electrogram demonstrates the earliest anterograde atrioventricular conduction at the left lateral free wall (CS 1, 2 area). B: atrioventricular reentrant tachycardia demonstrates the earliest retrograde ventriculoatrial conduction at the left lateral free wall (CS 1, 2 area). C: fluoroscopic images in the left anterior oblique (LAO) and right anterior oblique (RAO) projections successful demonstrating the site of the left lateral free wall bypass tract. RA: right atrium, His d: His distal, His m: His middle, His p: His proximal, CS: coronary sinus, RVA: right ventricular apex, ABL: ablation.
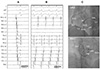
Fig. 3
Swallowing-induced, atrial tachycardias of two different types and successful ablation site. A: 12-lead surface electrocardiogram of two different inducible atrial tachycardias (ATs, AT1, and AT1). B: intra-cardiac electrogram recorded during swallowing. Two consecutive atrial premature complexes (APCs) followed by different morphologies of non-sustained AT (upper panel). Initially induced AT demonstrates the earliest atrial activation of the coronary sinus ostium (CS 9, 10 area) with a cycle length of 230 ms. (AT1, left lower panel). Another morphology of AT demonstrates the earliest atrial activation at the inferior-lateral wall of the tricuspid annulus (RA 5, 6 area) with a cycle length of 320 ms (AT2, right lower panel). C: successful ablation site of swallowing-induced atrial tachyarrhythmias. Electrogram showing the earliest atrial activation site at the left posterior interatrial septum (arrows), preceding the onset of P wave by 25 ms during APCs (left panel). Fluoroscopic images in the left anterior oblique (LAO) and right anterior oblique (RAO) projections, demonstrating the ablation catheter successful positioned at the site on the left posterior interatrial septum (right panel). RA: right atrium, His d: His distal, His m: His middle, His p: His proximal, CS: coronary sinus, RVA: right ventricular apex, ABL: ablation, ABL p: ablation proximal, ABL d: ablation distal.

References
1. Tada H, Kaseno K, Kubota S, et al. Swallowing-induced atrial tachyarrhythmias: prevalence, characteristics, and the results of the radiofrequency catheter ablation. Pacing Clin Electrophysiol. 2007; 30:1224–1232.
2. Greenspon AJ, Volosin KJ. Swallowing-induced tachycardia: electrophysiologic and pharmacologic observations. Pacing Clin Electrophysiol. 1988; 11(11 Pt 1):1566–1570.
3. Morady F, Krol RB, Nostrant TT, De Buitleir M, Cline W. Supraventricular tachycardia induced by swallowing: a case report and review of the literature. Pacing Clin Electrophysiol. 1987; 10(1 Pt 1):133–138.
4. Matsubara K, Inoue D, Morikawa Y, et al. Swallowing-induced arrhythmia. Clin Cardiol. 1988; 11:798–800.
5. Satish OS, Yeh SJ, Yeh KH, et al. Radiofrequency catheter ablation therapy of swallowing-induced atrioventricular nodal reentrant tachycardia: report of two cases. Pacing Clin Electrophysiol. 2005; 28:594–597.
6. Yeh SJ, Fu M, Lin FC, Chang CH, Hung JS. Paroxysmal supraventricular tachycardia initiated by a swallowing-induced premature atrial beat. J Electrocardiol. 1986; 19:193–196.
7. Baman NS, Baman TS, Taddonio W. Swallowing induced atrial fibrillation. Pacing Clin Electrophysiol. 2004; 27:555–556.
8. Yamauchi Y, Aonuma K, Sekiguchi Y, Higuchi K, Obayashi T, Isobe M. Curative therapy for swallowing-induced tachycardia by pulmonary vein antrum isolation. J Cardiovasc Electrophysiol. 2005; 16:1370–1374.
9. Yokoshiki H, Mitsuyama H, Watanabe M, Tsutsui H. Swallowing-induced multifocal atrial tachycardia originating from right pulmonary veins. J Electrocardiol. 2011; 44:395.e1–395.e5.
10. Undavia M, Sinha S, Mehta D. Radiofrequency ablation of swallowing-induced atrial tachycardia: case report and review of literature. Heart Rhythm. 2006; 3:971–974.
11. Nakahara S, Nagashima K, Okumura Y. Proximity relationship between epicardial adipose tissue and the endocardial origin of swallowing-induced atrial tachycardia. Heart Rhythm. 2014; 11:169–170.
12. Ardell JL. The cardiac neuronal hierarchy and susceptibility to arrhythmias. Heart Rhythm. 2011; 8:590–591.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download