Abstract
Background and Objectives
Heart failure (HF) patients display more varied QRS duration. We investigated whether QRS variability during hospitalization for acute decompensated HF is associated with poor clinical outcomes after discharge.
Subjects and Methods
One hundred seventy three patients (64% males; age 60±13 years) admitted for acute decompensated HF with severe left ventricular (LV) dysfunction (LV ejection fraction ≤35%) were consecutively enrolled. QRS variability was calculated by the difference between maximum and minimum QRS duration acquired during hospitalization. The prognostic implications on composite endpoints of death or urgent heart transplantation were analyzed.
Results
Forty-two patients (24.3%) died and three patients (1.7%) underwent urgent heart transplantation during the follow-up of 51±18 months. Patients who reached composite endpoints (n=45) showed greater QRS variability than those who did not (n=128) (20±23 ms vs. 14±14 ms, p=0.046). Patients who had high QRS variability (more than 22 ms; n=36) tended to have a higher event rate than those with QRS variability <22 ms {39% vs. 23%, hazard ratio (HR), 1.88; 95% confidence interval (CI) 1.001-3.539, p=0.05}. Adjusting with other variables, high QRS variability was an independent predictor for composite outcome (HR 1.94; 95% CI 1.023-3.683, p=0.042).
Heart failure (HF) continues to have a poor prognosis despite the advances in medical treatment.1) Previous studies suggested several predictors for mortality in HF population. QRS duration is a predictor of poor prognosis in HF.2)3) Wide QRS duration was found in 20-30% of HF patients, especially in those with severe left ventricular (LV) dysfunction.2)4)5) Patients with wide QRS duration display increased prevalence of sudden cardiac death and all-caused death.3)6-8) One of the possible explanations for poor outcomes in those with wide QRS duration is LV dyssynchrony.9) Cardiac resynchronization therapy could improve LV dyssynchrony and promote reverse remodeling, resulting in better outcomes in this high-risk population, especially those with wide QRS duration.10)11)
QRS duration can vary widely even within the same individual. HF patients are reported to have a wide QRS variation compared to those without HF, which changes maximally during HF exacerbation.12) A previous study reported on the prognostic value of QRS duration using electrocardiogram (ECG) on the day of admission and discharge for decompensated HF.13) There is little information whether the variation of QRS duration during HF exacerbation is related with prognosis. The change of QRS duration during HF exacerbation might occur from a transient or permanent non-specific intraventricular conduction delay and bundle branch block (BBB). Although BBB in HF patients shows poor clinical outcomes, few studies have addressed QRS variability and BBB in HF patients.13-16)
This study was undertaken to investigate whether QRS variation during hospitalization for acute decompensated HF is prognostically influential for clinical outcomes after discharge.
A total of 548 patients with LV ejection fraction (LVEF) ≤35% admitted for acute decompensated HF between December 2004 and April 2007 at Seoul National University Hospital (Seoul, Korea) were screened. Acute decompensated HF was defined as new onset or recurrence of gradually or rapidly developing symptoms and signs of HF requiring urgent or emergent therapy resulting in hospitalization.17) We selected 259 patients who had two or more ECGs during hospitalization. Among these patients, we excluded 10 without survival data and 59 with advanced malignancy with an expected survival of <6 months. Also, five patients with an implanted pacemaker or implantable cardioverter-defibrillator, or who underwent cardiac resynchronization therapy were excluded. Other exclusions included death (n=6) and heart transplantation (n=6) during index hospitalization. Finally, 173 patients were included for analysis.
We reviewed demographic variables, comorbidities, characteristics of admission, and biochemical data from the electrical medical records. Blood pressure, serum hemoglobin, serum creatinine, serum sodium, serum potassium, and serum B-type natriuretic peptide measured at admission day were included. Medications at admission and at discharge were reviewed. We also analyzed the presence of arrhythmia including atrial flutter (AFL), ventricular premature beat, or ventricular tachyarrhythmia during admission.
Electrocardiography performed within one month of admission was selected. Standard two-dimensional and color Doppler image triggered by the QRS complex were used for analysis. We collected left ventricular end diastolic dimension and LVEF using the M-mode.
We collected and reviewed all 12-lead ECGs performed during hospitalization (numbers of ECG, mean 7±4 per patient). All patients had their initial inpatient ECG within eight days of admission and 164 patients (95%) performed within one day after admission.
QRS duration was defined as the mean duration among all 12-leads as measured on a MAC1200 computer system (GE Healthcare, Chalfont St Giles, Buckinghamshire, UK). We selected maximum and minimum QRS duration value in each patient. Maximum QRS duration was defined as QRS duration with widest value and minimum QRS duration with narrowest value among whole ECG acquired during admission. QRS variability was defined as the difference between maximum and minimum QRS duration. Prolonged QRS duration was defined as a QRS duration exceeding 120 ms.3)8) High QRS variability was defined QRS variability more than 22 ms based on upper quintile value of QRS variability in study population. Fig. 1 depicts a representative case showing QRS variability.
To evaluate whether QRS duration in patients with atrial fibrillation (AF) or AFL varied in the present study, we measured QRS width manually in 10 consecutive beats and calculated mean duration. ECGs showing aberrant conduction due to rapid ventricular rate were not measured for analysis. QRS variability in patients with AF/AFL (n=61) was not different from those without (16.4±21.9 vs. 15.6±13.4, p=0.766).
We analyzed clinical outcome with composite endpoints of death or urgent heart transplantation.18)19) After discharge from index admission, we obtained follow-up data from hospital medical records and death certificates obtained from Ministry of Public Administration and Security of Korea. Patients who underwent heart transplantation during follow-up were censored on their transplantation date. Patients who survived without heart transplantation were censored in December 31, 2010.
Continuous variables were expressed as mean±standard deviation and categorical variables as numbers and percentages. Comparison of continuous variables was performed using Student's t-test or in case of non-normal distribution, Mann-Whitney U test when appropriate. Chi-square test was used for categorical variables. Event-free survival curves were constructed by the Kaplan-Meier survival curves and significance was tested with the log-rank test. The difference of each characteristic between two groups were analyzed by the hazard ratio (HR) and a 95% confidence interval (CI) using univariate Cox proportional hazard regression model. To evaluate the effects of the risk factors on composite endpoints, backward stepwise multivariate Cox regression analysis was performed. The variables with p<0.10 on univariate analysis were considered as candidate variables for multivariate analysis. Two-sided p<0.05 were considered statistically significant. Statistical Package for the Social Sciences software version 19.0 (IBM Corporation, Armonk, NY, USA) was used for all statistical analyses.
The baseline characteristics of study population are presented in Table 1. The 173 patients (110 males, 63.6%) had a mean age of 60±13 years. The mean follow-up duration was 51±18 months and 42 patients (24.3%) died and 3 patients (1.7%) underwent urgent heart transplantation during follow-up. One hundred seven patients (61.8%) and 66 (38.2%) were already diagnosed as HF and newly diagnosed as HF, respectively. The most common underlying etiology of HF was ischemic cardiomyopathy (n=84, 48.6%) and prevalence of acute myocardial infarction (AMI) on admission was 20% (n=35). Among 107 patients who were already diagnosed as HF before hospitalization, 54 (50.5%) patients were prescribed angiotensin converting enzyme inhibitors/angiotensin blockers and 30 (28%) patients received beta-blockers before index admission. Diuretics and aldosterone antagonists were prescribed in 49 (46%) and 20 (19%) patients, respectively. Medication history before index admission showed no significant difference between patients with or without composite endpoints events.
Patients had been admitted for mean 16±16 days (1 to 126 days). During admission, most patients (n=100, 57.8%) received conventional HF medical therapy only, whereas 21 patients (12.1%) underwent percutaneous coronary intervention and 26 patients (15.0%) received coronary artery bypass surgery. Twenty patients underwent valve operation; aortic valve replacement (n=7), mitral valve replacement (n=7), mitral valve plasty and mitral annuloplasty (n=3), dual valve replacement (n=2), and Bentall operation (n=1). One patient received AF ablation procedure during hospitalization.
Comparing the baseline characteristics according to composite outcome, the gender, comorbidities, medication at discharge, and prevalence of AMI on admission were similar in both groups. Patients with composite endpoints were older (64±11 years vs. 58±13 years, p=0.007), less newly diagnosed HF (20.0% vs. 44.5%, p=0.004), more ischemic cardiomyopathy (64.4% vs. 43.0%, p=0.013), less valvular heart disease (4.4% vs. 22.7%, p=0.006), and less valve operation (2.2% vs. 14.8%, p=0.023) than patients without composite endpoints. There were no significant differences between two groups in blood pressure, laboratory, and echocardiographic findings.
In ECG findings, 65% of patients were in sinus rhythm. There were no significant differences in heart rate, rhythm, and presence of arrhythmia in both groups. However, patients who reached composite endpoints showed significantly greater initial inpatient QRS duration (116±27 ms vs. 108±23 ms, p=0.049), last inpatient QRS duration (115±27 ms vs. 106±23 ms, p=0.028), maximum QRS duration (128±31 ms vs. 114±23 ms, p=0.006), and QRS variability (20±23 ms vs. 14±14 ms, p=0.046) than patients without composite endpoints.
The baseline characteristics of all subjects by QRS variability are summarized in Table 1. High QRS variability (QRS variability ≥22 ms) was observed in 36 patients (20.8%). Patients with high QRS variability showed greater maximum QRS duration (137±32 ms vs. 112±21 ms, p<0.001) and shorter minimum QRS duration (98±20 ms vs. 102±22 ms, p=0.260) than those with low QRS variability. High QRS variability group included more ischemic cardiomyopathy patients (61.1% vs. 45.3%, p=0.090) and less dilated cardiomyopathy patients (13.9% vs. 26.3%, p=0.120) than low QRS variability group without statistical significance. Patients with high QRS variability had longer hospital days (26±30 days vs. 13±9 days, p=0.021), more frequent ECG evaluation (10±5 times vs. 6±4 times, p<0.001), and higher prevalence of AMI on admission (33.3% vs. 16.8%, p=0.028).
Composite endpoints occurred more frequently in patients with high QRS variability than in patients with low QRS variability (38.9% vs. 22.6%, HR 1.882; 95% CI 1.001-3.539, p=0.050). Kaplan-Meier analysis revealed a better event-free survival rate among patients with low QRS variability (log-rank p=0.046) (Fig. 2).
Univariate Cox regression analysis showed that five of nine risk factors20)21) could be candidate predictors of an increased risk of composite endpoints; age, ischemic cardiomyopathy, serum hemoglobin levels, serum sodium levels, and high QRS variability, whereas prolonged initial inpatient QRS duration was not (Table 2). The unadjusted HR of high QRS variability for composite endpoints was 1.882 (95% CI 1.001-3.539, p=0.050). After adjustment with other significant variables, high QRS variability (HR 1.942, 95% CI 1.023-3.683, p=0.042), age (HR 1.030, 95% CI 1.005-1.057, p=0.020) and serum sodium levels (HR 0.935, 95% CI 0.875-0.999, p=0.046) were significant predictors of composite endpoints (Table 2).
Thirty-two (18.5%) patients presented BBB more than once during admission. According to the type of BBB, eight patients showed left BBB (LBBB) and 24 patients showed right BBB (RBBB). Patients with high QRS variability showed higher prevalence of BBB during admission than those with low QRS variability (41.7% vs. 12.4%, p<0.001). In patients with BBB, only five (14.3%) patients were AMI on admission. There was no relationship between presence of BBB and AMI on admission (p=0.472).
Composite endpoints were more frequent in the patients who presented BBB during admission than those without (46.9% vs. 21.3%, HR 2.46, 95% CI 1.32-4.57, p=0.008). The rate of composite endpoints was the highest in patients with LBBB (50.0%), intermediate in those with RBBB (45.8%), and the lowest in those without BBB (21.3%). RBBB was a statistically significant risk predictor of composite outcome (HR 2.17, 95% CI 1.10-4.29, p=0.022), while LBBB was not (HR 2.37, 95% CI 0.85-6.62, p=0.101).
There were three patients with bifascicular block. Presence of bifascicular block was not a statistically significant risk factor of composite endpoints (HR 2.36, 95% CI 0.57-9.73, p=0.237).
QRS variability measured during acute decompensated HF was an independent predictor of composite endpoints in HF patients with severe LV dysfunction.
QRS duration showed greater variability in HF patients than those without HF.12) Previous studies reported that prolongation of QRS duration (≥120 ms) occurs in approximately 30% among HF patients and is associated with increased risk of mortality.3) In consistent with previous reports, 33% of our study population showed prolonged QRS duration during hospitalization. Furthermore, the average of the mean QRS duration was more prolonged in the deceased group in our study.
Few studies have examined the change of QRS duration and its clinical significance in HF patients. Incremental changes of QRS duration could be related to progression of LV remodeling and intraventricular asynchrony, predicting more rapid progression to end state HF.22) We found that a wide variation of QRS duration including widening and shortening during acute decompensated state was an important predictor of poor clinical outcome in HF patients with severe LV dysfunction.
Wang et al.13) found that patients who developed prolonged QRS duration during hospitalization showed an increased risk of cardiovascular death compared to those with normal QRS duration. In addition, patients who presented with prolonged QRS duration at admission but who had normal QRS duration later during hospitalization still demonstrated an increased risk of mortality compared to patients with normal QRS duration on both initial and last ECG. In our study, patients who had high QRS variability but normal QRS duration (<120 ms) on their last inpatient ECG (n=25) showed a trend for worse clinical outcome of death or heart transplantation than patients who had low QRS variability and normal QRS duration in their last inpatients ECG (n=110, composite endpoints, 32.0% vs. 20.9%, p=0.234). Therefore, the change of QRS duration during hospitalization has more clinical implication than QRS duration itself in HF patients.
It is unclear why high QRS variability is related to worse clinical outcome. A failing heart undergoes electrical remodeling resulting increased PR interval and QRS duration.23) Also, metabolic abnormalities, myocardial ischemia or infarction, or pharmacological agents could aggravate conduction disturbance in HF. Prolonged QRS duration in HF patients is related to LV dyssynchrony.3) LV dyssynchrony could be reversed by cardiac resynchronization, resulting improvement of hemodynamic indices and long-term survival.24) Also, conduction delay in failing heart provides a substrate, such as scarred myocardium, for the development of ventricular tachyarrhythmia, and inducible ventricular tachycardia, suggesting the increased risk of sudden cardiac death.25) Though we could not define the cause of death in every individual patient, 44% died from progression of HF and 33% died from sudden cardiac arrest that could be associated with fatal arrhythmia among patients with known cause of death (n=18, 42.9% of deceased patients).
Most HF patients display incremental QRS duration during acute decompensation.12) However, whether the degree of QRS duration change has clinical significance is unclear. We hypothesized that QRS variability would be an early predictor of later QRS prolongation after discharge. QRS variability defined as a temporal fluctuation of QRS duration during acute decompensated state would predict QRS prolongation. Although QRS duration was normal at compensated state, the patients who showed high QRS variability might have had ventricular electrical and mechanical instability, rendering them prone to develop QRS prolongation in near feature.
Several studies have reported the relation of BBB to clinical outcome in HF patients.15) Whether the type of BBB is associated with outcome is debatable. Barsheshet et al.14) reported that RBBB has a 58% increased risk of 4-year mortality in patients with LVEF <30%. In the present study, RBBB was significantly associated with worsened clinical outcome, consistent with a previous study.14) However, the number of patients with LBBB in this study (n=8, 4.6%) might be insufficient to verify the impact of LBBB on clinical outcome.
Patients with high QRS variability received more ECG testing during admission (10±5 vs. 6±4, p<0.001) (Table 1). Patients who checked ECG frequently (more than five times during admission; n=88, 50.8%) showed higher QRS variability (19.9±15.2 ms vs. 11.8±17.6 ms, p=0.002). Also, the number of checked ECG and QRS variability showed significant correlation (Pearson's correlation coefficient=0.277, p<0.001). We analyzed whether the frequency of ECG test was related with severity. Although it was not possible to quantify the patient's severity due to lack of information, patients who received ECGs more than five times (n=88, 50.8%) showed longer hospital days (19±21 days vs. 13±8 days, p=0.022), higher prevalence of AMI on admission (35.2% vs. 4.7%, p<0.001), and received more percutaneous coronary intervention during admission (21.6% vs. 2.4%, p<0.001) than those who had less frequent ECG testing. Considering that more severe patients might perform more frequent ECG follow-up, high QRS variability and high number of ECG follow-up could be the factors that represent the severity of the patients. The number of performed ECG during admission did not show a significant difference between patients with and without composite endpoints (7±5 vs. 6±4, p=0.840).
Our study collected data retrospectively so that ECGs were performed without a prespecified schedule. Although the initial inpatient ECG was performed within 8 days, the time of last inpatient ECG varied. Only 53 patients (29%) had the final inpatient ECG within one day of discharge day. Although the final inpatient ECG might not have been the ECG at discharge, most of the final ECGs were recorded following recovery from decompensated HF. In addition, we could not collect detailed information on volume status, heart rate change during admission, and intravenously administered pharmacologic agents, which can influence QRS duration. We could also not analyze serum magnesium levels, which could affect QRS duration, because the levels were measured only in 14 patients.
Although the population of our study included various causes of HF, analysis according to their etiologies was limited. QRS variability was measured during acute decompensated period; therefore it would be different with QRS duration measure in stable condition. Therefore, the results of our study should be carefully interpreted into specific condition rather than general HF patients.
QRS variability during hospitalization for acute decompensated HF was an independent predictor for poor clinical outcome in HF patients with severely reduced LVEF. Therefore, patients who present high QRS variability during acute decompensation may regard as high risk population for death or heart transplantation. Further investigations are needed to apply the results of the present study to general HF patients.
Figures and Tables
Fig. 1
Representative QRS variability calculation. A: shows initial QRS duration (144 ms) taken on admission day. B: after HF treatment (hospital day 17), QRS duration became shortened to 108 ms. QRS variability was 36 ms. This patient has died of unknown cause during 20 months follow-up. HF: heart failure, ECG: electrocardiogram.
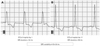
Fig. 2
Kaplan-Meier analysis of composite endpoints according to QRS variability. Patients with marked QRS variability showed higher event-rate than those with low QRS variability.

Table 1
Baseline characteristics of study patients categorized by QRS variability
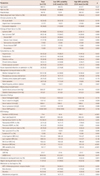
Table 2
Cox regression analysis for composite endpoints

Acknowledgments
This study was supported by grant number 03-2011-0370, 04-2010-0260, 04-2010-1140 from the Seoul National University Hospital research fund.
References
1. Cleland JG, Gemmell I, Khand A, Boddy A. Is the prognosis of heart failure improving? Eur J Heart Fail. 1999; 1:229–241.
2. Shenkman HJ, Pampati V, Khandelwal AK, et al. Congestive heart failure and QRS duration: establishing prognosis study. Chest. 2002; 122:528–534.
3. Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005; 46:2183–2192.
4. Murkofsky RL, Dangas G, Diamond JA, Mehta D, Schaffer A, Ambrose JA. A prolonged QRS duration on surface electrocardiogram is a specific indicator of left ventricular dysfunction. J Am Coll Cardiol. 1998; 32:476–482.
5. Sandhu R, Bahler RC. Prevalence of QRS prolongation in a community hospital cohort of patients with heart failure and its relation to left ventricular systolic dysfunction. Am J Cardiol. 2004; 93:244–246.
6. Brilakis ES, Mavrogiorgos NC, Kopecky SL, et al. Usefulness of QRS duration in the absence of bundle branch block as an early predictor of survival in non-ST elevation acute myocardial infarction. Am J Cardiol. 2002; 89:1013–1018.
7. Kalra PR, Sharma R, Shamim W, et al. Clinical characteristics and survival of patients with chronic heart failure and prolonged QRS duration. Int J Cardiol. 2002; 86:225–231.
8. Iuliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. Department of Veterans Affairs Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. QRS duration and mortality in patients with congestive heart failure. Am Heart J. 2002; 143:1085–1091.
9. Bleeker GB, Schalij MJ, Molhoek SG, et al. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004; 15:544–549.
10. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005; 352:1539–1549.
11. Sipahi I, Carrigan TP, Rowland DY, Stambler BS, Fang JC. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch Intern Med. 2011; 171:1454–1462.
12. Aranda JM, Carlson ER, Pauly DF, et al. QRS duration variability in patients with heart failure. Am J Cardiol. 2002; 90:335–337.
13. Wang NC, Maggioni AP, Konstam MA, et al. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008; 299:2656–2666.
14. Barsheshet A, Goldenberg I, Garty M, et al. Relation of bundle branch block to long-term (four-year) mortality in hospitalized patients with systolic heart failure. Am J Cardiol. 2011; 107:540–544.
15. McCullough PA, Hassan SA, Pallekonda V, et al. Bundle branch block patterns, age, renal dysfunction, and heart failure mortality. Int J Cardiol. 2005; 102:303–308.
16. Baldasseroni S, Gentile A, Gorini M, et al. Intraventricular conduction defects in patients with congestive heart failure: left but not right bundle branch block is an independent predictor of prognosis. A report from the Italian Network on Congestive Heart Failure (IN-CHF database). Ital Heart J. 2003; 4:607–613.
17. Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009; 53:557–573.
18. Lee CW, Lee JH, Lim TH, et al. Prognostic significance of cerebral metabolic abnormalities in patients with congestive heart failure. Circulation. 2001; 103:2784–2787.
19. Gulati A, Ismail TF, Jabbour A, et al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation. 2013; 128:1623–1633.
20. Parameshwar J, Keegan J, Sparrow J, Sutton GC, Poole-Wilson PA. Predictors of prognosis in severe chronic heart failure. Am Heart J. 1992; 123:421–426.
21. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005; 112:e154–e235.
22. Shamim W, Yousufuddin M, Cicoria M, Gibson DG, Coats AJ, Henein MY. Incremental changes in QRS duration in serial ECGs over time identify high risk elderly patients with heart failure. Heart. 2002; 88:47–51.
23. Xiao HB, Roy C, Fujimoto S, Gibson DG. Natural history of abnormal conduction and its relation to prognosis in patients with dilated cardiomyopathy. Int J Cardiol. 1996; 53:163–170.
24. Leclercq C, Cazeau S, Le Breton H, et al. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J Am Coll Cardiol. 1998; 32:1825–1831.
25. Horwich T, Lee SJ, Saxon L. Usefulness of QRS prolongation in predicting risk of inducible monomorphic ventricular tachycardia in patients referred for electrophysiologic studies. Am J Cardiol. 2003; 92:804–809.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download