Abstract
Background and Objectives
Hyperuricemia is known to be a risk factor for atherosclerosis, as is gender. The variables related to metabolic syndrome (MS), as well as other cardiovascular risk factors such as serum uric acid (SUA), differ according to gender. The aim of this study was to evaluate the relationship between SUA and the variables of MS according to gender.
Subjects and Methods
We randomly recruited 675 subjects (373 men and 302 women), who underwent health screening. The subjects were divided into four groups according to SUA quartiles. We compared each quartile of the SUA with the incidence of MS. The variables included body mass index (BMI), hypertension, fasting blood glucose (FBS), high-density lipoprotein (HDL) cholesterol, triglyceride (TG), and the MS score.
Results
The incidence of MS in men was significantly increased compared to women, and the incidence of MS was increased according to the SUA values in women. The MS scores tended to increase according to the SUA values in both genders. The incidence of high BMI, high blood pressure, and high TG were correlated with the SUA values in both genders. However, HDL-cholesterol was correlated with MS scores in women, and fasting glucose was not correlated with MS in either gender.
Figures and Tables
Fig. 4
Relationship between low high-density lipoprotein (HDL) cholesterol and serum uric acid (SUA) quartiles.
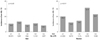
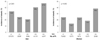
Fig. 8
Relationship between metabolic syndrome and serum uric acid (SUA) quartiles according to menopause.

Table 2
Pearson's correlation coefficients for the relationship between variables of metabolic syndrome and serum uric acid in total person

Table 3
Pearson's correlation coefficients for the relationship between variables of metabolic syndrome and serum uric acid in men and women

References
1. Costa A, Iguala I, Bedini J, Quinto L, Conget I. Uric acid concentration in subjects at risk of type 2 diabetes mellitus: relationship to components of the metabolic syndrome. Metabolism. 2002. 51:372–375.
2. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003. 52:1210–1214.
3. Cha BS, Kim HJ. Metabolic syndrome and cardiovascular disease. Korean Circ J. 2003. 33:645–652.
4. Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006. 95:136–147.
5. Curb JD, Ford C, Hawkins CM, et al. A coordinating center in a clinical trial: the hypertension detection and followup program. Control Clin Trials. 1983. 4:171–186.
6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001. 285:2486–2497.
7. Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003. 9:237–252.
8. Park HS, Oh SW, Kang JH, et al. Prevalence and associated factors with metabolic syndrome in South Korea: from the Korean National Health and Nutrition Examination Survey, 1998. J Korean Soc Study Obes. 2003. 12:1–14.
9. International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. 2000. Western Pacific Region.
10. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant-and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981. 78:6858–6862.
11. Yoo TW, Sung KC, Kim YC, et al. The relationship of the Hypertension, insulin resistance, and metabolic syndrome in the serum uric acid level. Korean Circ J. 2004. 34:874–882.
12. Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet. 1999. 354:650.
13. Waring WS, Adwani SH, Breukels O, Webb DJ, Maxwell SR. Hyperuricaemia does not impair cardiovascular function in healthy adults. Heart. 2004. 90:155–159.
14. Alderman MH, Cohen H, Madhavan S, Kinlighn S. Serum uric acid and cardiovascular events in successfuolly treated hypertensive patients. Hypertension. 1999. 34:144–150.
15. Sowers JR, Lester MA. Diabetes and cardiovascular disease. Diabetes Care. 1999. 22:Suppl 3. C14–C20.
16. Kim JH, Moon HS. Health perception, body image, sexual function and depression in menopausal women according to menopausal stages. J Korean Acad Nurs. 2006. 36:449–456.
17. Jitapunkul S, Chalapraprawat M, Bunnag S, et al. The relationship between glucose and uric acid metabolism: influence of short term allopurinol on glucose metabolism. J Med Assoc Thai. 1991. 74:80–86.
18. Yun JW, Kang JM, Lee WY, Kim SW. Plasma uric acid concentrations represent the degree of metabolic control and diabetic complications in type 2 diabetes. Korean J Med. 2003. 64:78–84.




 PDF
PDF ePub
ePub Citation
Citation Print
Print










 XML Download
XML Download