Abstract
Purpose
Mesenchymal stem cells (MSCs) are surfacing as a new method of treatment for various diseases that have poor outcome with drug treatments. In this study, we investigated the effects of MSCs in a murine intestinal inflammation model mimicking human Crohn's disease (CD) using 2,4,5-trinitrobenzene sulfonic acid (TNBS).
Methods
Colitis was induced by rectal administration of 2 mg of TNBS in 35% ethanol as experimental group compared to control group. Histological changes, surface molecules of T and B cells of the spleen and blood, and cytokine production (IFN-γ, TNF-α, IL-4, IL-6, IL-10, and IL-12) were determined among 3 groups comprised of control group, TNBS group and TNBS/MSC group.
Results
In the mice treated with MSCs, there was a decrease in the wasting disease process and inflammatory histopathological changes. There was also a decrease in pro-inflammatory T-helper 1 (Th1) cytokines IFN-γ and IL-12 and T-helper 2 (Th2) cytokine IL-4. Anti-inflammatory cytokine IL-10 increased in mice treated with MSCs compared to colitic mice. The blood CD4+CD25+ T-regulatory cells also increased and splenic CD19 B-cells decreased.
Inflammatory bowel disease (IBD) is defined as chronic inflammation of the bowel of unknown etiology and normally refers to Crohn's disease (CD) and ulcerative colitis. While specific causes are unknown, it is thought to be associated with the bowel's immunological function, especially activated T-lymphocytes.(1,2) T-lymphocytes secrete cytokines which regulate immune response and according to their function, are divided into T-helper 1 (Th1) (IL-2, interferon-γ; regulation of cell-mediated immunity) and T-helper 2 (Th2) (IL-4, IL-5, IL-10, IL-13; regulation of humoral immunity) cells.(3,4) Dysregulation of T-lymphocytes in IBD leads to imbalance of these cytokines, causing an influx of inflammatory cells such as T-lymphocytes and macrophages and subsequent tissue damage.(5) Also, genetic and psychological susceptibility, altered bowel microflora and various other factors are thought to be involved in the inflammatory process.(6,7) IBD progresses either gradually over a long period or acutely over several days.(8) With lack of a definitive treatment option due to an ill-defined etiology, most patients enter a chronic disease state.(2)
Current medical treatment for IBD mainly consists of pharmacological intervention. Rather than achieving a cure, the current aim of treatment is to improve quality of life by inducing remission, preventing recurrence and controlling the symptoms.(9) Drugs used include aminosalicylates (sulfasalazine, mesalamine), corticosteroid-based anti-inflammatories, antibiotics (metronidazole), immune boosters (levamisole, Bacillus Calmette Guerin (BCG)), a mast cell stabilizer (cromolyn sodium), and allopathic medications including antidiarrheals, antispasmodics and cholestyramine.(9,10) An immunosuppressant (azathioprine, mercaptopurine, methotrexate, cyclosporine) can be used when other drug treatments are ineffective.(10) However, many of these drugs face limited use because of ineffectiveness and adverse side effects.
Due to issues facing drug treatments, treatment using mesenchymal stem cells (MSC) is surfacing as a new method of treatment. MSCs are multipotent non-hematopoietic progenitor cells from adult bone marrow which can differentiate into various cells that make up fat, cartilage, bone, muscle, stroma, nerves, etc.(11) Currently, clinical research is aimed towards achieving tissue regeneration with MSCs.
MSCs have a low expression of antigens such as HLA-DR, and therefore produce less adverse immune responses when injected in vivo.(12,13) MSCs can be genetically modified, and they can produce various growth factors or cytokines continuously or for certain periods of time when injected.(14) They cannot present antigen due to absent MHC-II and co-stimulatory molecules, but they play an important role in proliferation and growth of hematopoietic stem cells and produce various cytokines and regulatory molecules that regulate granulocyte, macrophage and dendritic cell production, hemopoiesis and immune response.(11,12)
MSCs with low immunogenicity, immunomodulatory function, and ability to differentiate into various cell lineages have not shown graft versus host disease or caused damage to the recipient's immune system.(15,16) Also, by forming chimeras, they have led to donor-specific immune tolerance.(17)
Cytotherapy using stem cells is receiving attention as the next generation treatment method as it can be used in rejection or incurable illnesses that current medicines cannot solve.(12) Cytotherapy also can be applied to various fields of medicine including cardiovascular, neurological, hematological, and immunological systems, and also hereditary, hepatic and endocrine diseases. Utilization of MSCs in the treatment of IBD requires more in-depth research to clarify its mechanism of action. Therefore, in the present study, we examined the effect of MSCs in the murine model of TNBS-induced colitis.
Female 8-week-old BALB/c mice (weight range 20~25 g) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All mice were housed in specific pathogen-free conditions and in accordance with institutional guidelines. Colitis was induced by rectal administration of 2 mg of 2,4,6-trinitrobenzene sulfonic acid (TNBS, Sigma Chemical Co., St. Louis, MO, USA) in 35% ethanol (Merck, Germany) using a vinyl catheter positioned 4 cm from the anus (20 mice per group). After instillation they were kept vertically for 60 seconds with mice faced down for effective absorption and prevention of regurgitation of administered TNBS or ethanol. The MSC treatment group was treated with 5×105 cells/150 µl in Dulbecco's Phosphate Buffered Saline (DPBS, GIBCO, NY, USA) isolated from the mice inguinal fat pad, via tail vein 24 hrs after colitis induction. The control group was treated with 35% ethanol (Et-OH).
Mice were weighed daily. Body weight loss was determined by percentage of weight loss from baseline body weight. Mice were sacrificed on each 3 (n=10) and 10 days (n=10) after the TNBS administration. The longitudinally divided colons were rolled up and fixed in 4% buffered formalin. Fixed tissues were embedded in paraffin, and 4~6 µm sections were stained with H&E for histologic grading. An experienced pathologist blinded to treatment allocation scored all sections: (1) percentage of colon involved, (2) infiltration of mononuclear cells, and (3) alteration & erosion. The severity was scored on a scale of 0~4 as follows: 0, absent; 1, weak; 2, moderate; 3, severe; 4, most severe. Grading was done in a blinded fashion by one of the authors.
The surface molecules of T cells were observed in the peripheral blood and spleen by the use of mAb directed to CD4, CD8a, CD19, CD25 and a control isotype conjugated with fluorochromes (FITC, PE). All Abs were purchased from BD PharMingen (San Diego, CA, USA) and analysis of the samples was carried out on a FACS Vantage flow cytometer (BD Biosciences, USA). Splenocytes were cultured in 200 µl of RPMI 1640 medium supplemented with L-glutamine, antibiotics, 10% FBS and 2 µg/ml anti-CD28 mAb in 96-well plates (Costar, USA) precoated with 5 µg/ml anti-CD3 mAb for 72 hrs. Cytokine concentrations in the culture supernatants were determined by specific ELISA according to the manufacturer's instructions (R&D system, USA).
To investigate the role of MSC in experimental colitis and the therapeutic effect of MSC, colitis was induced in BALB/c mice. Colitis was induced by intrarectal administration of TNBS, which haptenates autologous colonic proteins with trinitrophenol. Administration of TNBS resulted in diarrhea and wasting disease with an increased mortality rate of approximately 60% after 10 days (Fig. 1). TNBS colitic mice treated with MSCs showed a markedly increased survival rate of 90% compared to TNBS colitic mice of 40% (Fig. 1).
Similar decrease in body weight was observed in both TNBS colitic mice and MSC treated colitic mice at day 1 (Fig. 2). Decrease in body weight (maximum of more than 10%) was observed until day 3 followed by gradual recovery in TNBS colitic mice (Fig. 2). However, MSC treated colitic mice showed no further weight loss after day 1 and showed rapid weight recovery (Fig. 2).
Thus, MSC treatment in colitis-induced mice resulted in increased survival rate and decreased weight loss.
Induction of TNBS colitis resulted in a slight decrease in CD4+ T cells in the blood and spleen (except day 10 in spleen), whereas MSC treatment showed increase in it. CD8a+ T cells showed a slight decrease after TNBS treatment in both blood and spleen samples, but increased after MSC treatment. CD19+ T cells in the spleen showed a 30~40% decrease in the TNBS group compared to control/Et-OH group, but showed a dramatic decrease after MSC treatment, and CD4+CD25+ T cells showed a marked increase in TNBS/MSC group compared to TNBS group in the blood on day 3 (Table 2).
In order to determine the treatment effect of MSC in TNBS colitis, splenocytes producing various cytokines were cultured and ELISA was performed.
Concentrations of IFN-γ and IL-12 from the Th1 group (IFN-γ, TNF-α, IL-12) were significantly lower in the MSC treated group compared to the control (Et-OH) and TNBS groups, and concentrations of TNF-α were significantly higher in the MSC treated group compared to the TNBS group on day 3 but markedly decreased by day 10 which showed that there is a decreasing effect on pro-inflammatory markers (Fig. 4).
Concentration of IL-4 in the Th2 group (IL-4, IL-6, IL-10) was significantly lower in the MSC treated group compared to the TNBS group on day 10. In contrast, the concentration of IL-6 was significantly higher in the MSC treated group on day 3. The concentration of IL-10 on day 3 showed no noticeable differences in all 3 groups, but the MSC treated group showed a significant increase on day 10 compared to the TNBS group (Fig. 4).
In this study, we examined the effects of MSC on TNBS-induced colitis, which is a CD4+ T-cell mediated autoimmune disease.
Clinical results from H&E staining showed a significant decrease in both the microscopic inflammatory process and in damage in the TNBS/MSC group compared to the TNBS group. On day 10, all of the involved regions showed a significant decrease in alteration and erosion. Mononuclear cell infiltration was also decreased, but was not statistically significant. These are all signs of a decrease in the inflammatory process, which proves that MSC has an anti-inflammatory effect on microscopic inflammation.
IFN-γ and IL-12 decreased in the TNBS/MSC group compared to the TNBS group, which showed a decrease in Th1 response. IFN-γ and IL-12 both significantly decreased on both days 3 and 10. This is possibly due to termination of the IFN-γ - IL-12 - IFN-γ - IL-12 loop by MSC. In this loop, increase in IFN-γ leads to an increase in IL-12, which itself causes the IFN-γ to increase again in a loop.(18) This illustrates the Th1 polarization (decrease in Th1 response that is responsible for cellular immunity) and inhibition of inflammation that is triggered by the inflammatory IFN-γ cytokine.
IL-4 decreased and IL-10 increased in the TNBS/MSC group compared to the TNBS group, which showed an anti-inflammatory response in the Th2-mediated immune response. IL-4 acts on the T-helper 0 (Th0) cells to differentiate into Th2 cells.(19) Subsequently, IL-4 acts on Th2 cells to produce Th2 cytokines such as IL-5, IL-6, IL-13 and even IL-4 (paracrine effect).(20,21). Therefore, a significant decrease in IL-4 cells attenuates the Th2 immune response, leading to decreased humoral immunity. Also, IL-4 works with IFN-γ, where an increase in IFN-γ also leads to a subsequent increase in IL-4.(19) IL-10 also antagonizes the production of Th1 cytokines IFN-γ and IL-12.(22) Therefore, the significant decrease in IFN-γ correlates with the significant decrease in IL-4 and the significant increase in IL-10, showing that the TNBS/MSC group is effective in attenuating the inflammatory process. Aggarwal and Pittenger(14) also reported a decrease in IFN-γ and an increase in IL-10, but also an increase in IL-4 in their study using human MSC, which showed a Th2 shift. Our current results might suggest a general decrease in both Th1 and 2 inflammatory processes by MSC. In addition, the significant increase in IL-6 level in day 3 TNBS/MSC group is explainable as IL-6 causes increased migration and activation of MSC.(23)
Cell surface markers from the TNBS/MSC group also showed the anti-inflammatory effect of MSC. A significant increase in CD4+CD25+ T cells in the blood sample of the TNBS/MSC group showed that T-regulatory cells that attenuate the inflammatory process increased.(24,25) This is in line with the study by Veltkamp et al.(26), which had shown that CD4+CD25+ T-regulatory cells prevent experimental colitis in the bone marrow transplanted murine model. Also, a significant decrease in CD19 in the spleen sample of the TNBS/MSC group on day 10 showed effective attenuation of the Th2 response as CD19+ B cell subset population blocks T regulatory cells and exacerbates ileitis in murine model of CD.(27,28) However, the general decrease in CD19 level in the TNBS group compared to the control group requires further research.
However, cell surface markers from the TNBS group yielded different results. CD8a (blood, day 4) and CD4 (blood and spleen, day 10) in the TNBS/MSC group all showed a significant increase compared to the TNBS group, which shows that there is still inflammation and a Th1 response. This could have been due to a remaining inflammatory process that was not attenuated by MSCs.
Also, TNF-α levels did not correlate with other cytokine levels. TNF-α levels significantly increased on day 3 in the TNBS/MSC group compared to the TNBS group, which shows that there is a significant inflammatory process present. This result did not correlate with other cytokine levels which all showed a decrease, signifying a decreased inflammatory process. Whereas this study showed no significant decrease in the TNF-α levels by MSCs, Lee et al.(29,30) showed that DSG and anti-4-1 BB mAb leads to a decrease in TNF-α levels. However, TNF-α significantly increased only on day 3 but not on day 10 in this study, which can be interpreted as MSCs working more effectively in the later part of the inflammation process and not in the earlier part. Also, results from H&E staining showed a significant decrease in the microscopic inflammatory process on day 10 which correlates to the lack of a significant rise in these cytokine levels by then.
There is much research being done on the use of MSCs in treating intestinal diseases. It has been shown that adipose-derived MSCs can be used to treat rectovaginal fistulas in perianal CD without any adverse effects.(31) Research has suggested a great potential for the application of MSC. However, the mechanism of action still requires more in depth clarification, which will provide the direction and focus for future study. The current study would have been able to yield more reliable results if the sample sizes were larger. The addition of an apoptosis detection assay to monitor the decrease in specific cell populations would also have given more accurate findings. In addition, monitoring other cytokines such as CD11 (leukocyte adhesion, macrophage levels) would have given more insight into the anti-inflammatory process. Research comparing the effect of using MSC treatment along with conventional treatment versus conventional treatment alone could be meaningful. Because of MSC's immunomodulatory effect, synergy with conventional treatment to increase treatment effectiveness and decrease side effects can be expected.
In conclusion, MSC's immunomodulatory effect in itself was shown to have a possible therapeutic effect in the murine IBD model, especially in CD. With further research, it could be possible to utilize MSC in the clinical treatment of IBD if subsequent experiments can prove the safety of MSC therapy.
Figures and Tables
Fig. 1
Survival rate and body weight after induction of TNBS colitis. Survival rate after induction of TNBS colitis in mice treated with MSC. Application of MSC caused a significant decrease in mortality rates in TNBS colitis. The graph represents data from six independent experiments with at least ten mice in each group.

Fig. 2
TNBS colitis and influence of MSC on body weight. Body weight was recorded daily from days 1 to 10. The change in weight is expressed as the percentage of body weight from day 1. Administration of MSC reduced weight loss in TNBS colitis. Results are representative of six experiments.

Fig. 3
Histological analysis of colonic specimens on day 3 and 10 after induction of TNBS colitis. (A) Control/Et-OH mice post-op day 3 (A-1) and day 10 (A-2), respectively. (B) TNBS colitis induced mice post-op day 3 (B-1) and day 10 (B-2), respectively. (C) TNBS colitis induced mice treated with MSCs post-op day 3 (C-1) and day 10 (C-2). Treatment with MSCs led to reduction in TNBS-induced inflammation. Significantly less inflammation, fewer cell infiltration and crypt destruction were present in MSC treated colitic mice (C) compared with TNBS colitic mice (B).
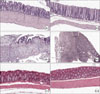
Fig. 4
Effect of MSC treatment on cytokine production. The results are representative of six experiments. *P<0.05.

References
1. Podolsky DK. Inflammatory bowel disease (1). N Engl J Med. 1991. 325:928–937.
2. Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998. 115:182–205.
3. MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000. 51:2–9.
4. Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001. 120:622–635.
5. Geboes K. From inflammation to lesion. Acta Gastroenterol Belg. 1994. 57:273–284.
6. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003. 3:521–533.
7. Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003. 3:331–341.
8. Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007. 117:514–521.
9. Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002. 122:1592–1608.
10. Van Deventer SJ. Immunotherapy of Crohn's disease. Scand J Immunol. 2000. 51:18–22.
11. Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007. 262:509–525.
12. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–48.
13. Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003. 10:228–241.
14. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005. 105:1815–1822.
15. Frank MH, Sayegh MH. Immunomodulatory functions of mesenchymal stem cells. Lancet. 2004. 363:1411–1412.
16. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004. 363:1439–1441.
17. Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, et al. Allogeneic bone marrow-derived flk-1+Sca-1-mesenchymal stem cells leads to stable mixed chimerism and donor-specific tolerance. Exp Hematol. 2004. 32:861–867.
18. Ertel W, Keel M, Neidhardt R, Steckholzer U, Kremer JP, Ungethuem U, et al. Inhibition of the defense system stimulating interleukin-12 interferon-gamma pathway during critical illness. Blood. 1997. 89:1612–1620.
19. Miner KT, Croft M. Generation, persistence, and modulation of Th0 effector cells: role of autocrine IL-4 and IFN-gamma. J Immunol. 1998. 160:5280–5287.
20. Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993. 362:245–248.
21. Chen L, Grabowski KA, Xin JP, Coleman J, Huang Z, Espiritu B, et al. IL-4 induces differentiation and expansion of Th2 cytokine-producing eosinophils. J Immunol. 2004. 172:2059–2066.
22. Osugi Y, Hara J, Tagawa S, Takai K, Hosoi G, Matsuda Y, et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997. 89:4100–4103.
23. Rattigan Y, Hsu JM, Mishra PJ, Glod J, Banerjee D. Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp Cell Res. Epub 2010 Jul 13.
24. Stephens LA, Gray D, Anderton SM. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc Natl Acad Sci U S A. 2005. 102:17418–17423.
25. Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004. 16:89–98.
26. Veltkamp C, Sartor RB, Giese T, Autschbach F, Kaden I, Veltkamp R, et al. Regulatory CD4+CD25+ cells reverse imbalances in the T cell pool of bone marrow transplanted TGepsilon26 mice leading to the prevention of colitis. Gut. 2005. 54:207–214.
27. Gärdby E, Chen XJ, Lycke NY. Impaired CD40-signalling in CD19-deficient mice selectively affects Th2-dependent isotype switching. Scand J Immunol. 2001. 53:13–23.
28. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006. 8:Suppl 2. S3.
29. Lee J, Kim MS, Kim EY, Park HJ, Chang CY, Jung DY, et al. 15-deoxyspergualin prevents mucosal injury by inhibiting production of TNF-alpha and down-regulating expression of MD-1 in a murine model of TNBS-induced colitis. Int Immunopharmacol. 2007. 7:1003–1012.
30. Lee J, Lee EN, Kim EY, Park HJ, Chang CY, Jung DY, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of inflammatory bowel disease. Immunol Lett. 2005. 101:210–216.
31. García-Olmo D, García-Arranz M, García LG, Cuellar ES, Blanco IF, Prianes LA, et al. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn's disease: a new cell-based therapy. Int J Colorectal Dis. 2003. 18:451–454.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download