Abstract
Insulin resistance is frequently associated with chronic liver disease, and the interaction between hepatitis C virus (HCV) infection and insulin resistance is a major public health issue, bound to increase in the near term. Because of their potential synergism on liver disease severity, a better understanding of the clinical consequences of the relationship between HCV infection and insulin resistance is needed. This translates into accelerated liver disease progression, reduced response to anti-viral agents and, in susceptible individuals, increased risk of developing type 2 diabetes. HCV may also cause hepatic steatosis, especially in patients infected with genotype 3, although the clinical impact of viral steatosis is debated. Little is known regarding the effect of anti-diabetic agents on HCV infection, and a possible association between use of exogenous insulin or a sulfonylurea agents and the development of hepatocellular carcinoma has recently been reported. Thus, modified lifestyle and pharmacological modalities are urgently warranted in chronic hepatitis C with metabolic alterations.
Figures and Tables
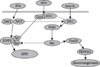
Fig. 1
Schematic representation of expected interaction between interferon and insulin at the presence of hepatitis C virus. Dotted lines represent the facts that need more to be identified.
HCV, hepatitis C virus; IFN, interferon; IRS, insulin receptor substrates; PI3K, phosphoinositide 3-kinase; FoxO1, forkhead box protein O 1; PEPCK1, phosphoenolpyruvate carboxykinase 1; JAK1, janus kinase 1; Tyk2, tyrosine kinase 2; STAT1, signal transducers and activators of transcription family of transcription factors 1; Tyr, tyrosine; Ser, serine; ISRE, interferon-sensitive response.
References
1. Bartenschlager R, Ahlborn-Laake L, Mous J, Jacobsen H. Kinetic and structural analyses of hepatitis C virus polyprotein processing. J Virol. 1994. 68:5045–5055.
2. Grakoui A, Wychowski C, Lin C, Feinstone SM, Rice CM. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993. 67:1385–1395.
3. Bugianesi E, Salamone F, Negro F. The interaction of metabolic factors with HCV infection: does it matter? J Hepatol. 2012. 56:Suppl 1. S56–S65.
4. Ferrannini E, Natali A, Capaldo B, Lehtovirta M, Jacob S, Yki-Järvinen H. European Group for the Study of Insulin Resistance (EGIR). Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity. Hypertension. 1997. 30:1144–1149.
5. Harrison SA. Insulin resistance among patients with chronic hepatitis C: etiology and impact on treatment. Clin Gastroenterol Hepatol. 2008. 6:864–876.
6. Romero-Gómez M. Insulin resistance and hepatitis C. World J Gastroenterol. 2006. 12:7075–7080.
7. Douglas MW, George J. Molecular mechanisms of insulin resistance in chronic hepatitis C. World J Gastroenterol. 2009. 15:4356–4364.
8. Lam KD, Bacchetti P, Abbasi F, et al. Comparison of surrogate and direct measurement of insulin resistance in chronic hepatitis C virus infection: impact of obesity and ethnicity. Hepatology. 2010. 52:38–46.
9. Negro F. Abnormalities of lipid metabolism in hepatitis C virus infection. Gut. 2010. 59:1279–1287.
10. Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000. 133:592–599.
11. Mehta SH, Brancati FL, Strathdee SA, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003. 38:50–56.
12. Chen T, Jia H, Li J, Chen X, Zhou H, Tian H. New onset diabetes mellitus after liver transplantation and hepatitis C virus infection: meta-analysis of clinical studies. Transpl Int. 2009. 22:408–415.
13. Fabrizi F, Messa P, Martin P, Takkouche B. Hepatitis C virus infection and post-transplant diabetes mellitus among renal transplant patients: a meta-analysis. Int J Artif Organs. 2008. 31:675–682.
14. Moucari R, Asselah T, Cazals-Hatem D, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008. 134:416–423.
15. Simó R, Lecube A, Genescà J, Esteban JI, Hernández C. Sustained virological response correlates with reduction in the incidence of glucose abnormalities in patients with chronic hepatitis C virus infection. Diabetes Care. 2006. 29:2462–2466.
16. Arase Y, Suzuki F, Suzuki Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009. 49:739–744.
17. Kawaguchi T, Ide T, Taniguchi E, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007. 102:570–576.
18. Douglas MW, George J. Molecular mechanisms of insulin resistance in chronic hepatitis C. World J Gastroenterol. 2009. 15:4356–4364.
19. Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004. 165:1499–1508.
20. Pazienza V, Clément S, Pugnale P, et al. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007. 45:1164–1171.
21. Persico M, Russo R, Persico E, et al. SOCS3 and IRS-1 gene expression differs between genotype 1 and genotype 2 hepatitis C virus-infected HepG2 cells. Clin Chem Lab Med. 2009. 47:1217–1225.
22. Chung WJ, Sabharwal S, Tai AW, et al. HCV induces insulin resistance by decreasing throsine IRS1 phosphorylation in JFH1 infected hepatocytes. Hepatology. 2009. 50:Suppl 4. 938A.
23. Pazienza V, Vinciguerra M, Andriulli A, Mangia A. Hepatitis C virus core protein genotype 3a increases SOCS-7 expression through PPAR-{gamma} in Huh-7 cells. J Gen Virol. 2010. 91:1678–1686.
24. Bernsmeier C, Duong FH, Christen V, et al. Virus-induced over-expression of protein phosphatase 2A inhibits insulin signalling in chronic hepatitis C. J Hepatol. 2008. 49:429–440.
25. Duong FH, Christen V, Berke JM, Penna SH, Moradpour D, Heim MH. Upregulation of protein phosphatase 2Ac by hepatitis C virus modulates NS3 helicase activity through inhibition of protein arginine methyltransferase 1. J Virol. 2005. 79:15342–15350.
26. Ugi S, Imamura T, Maegawa H, et al. Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol Cell Biol. 2004. 24:8778–8789.
27. Miyamoto H, Moriishi K, Moriya K, et al. Involvement of the PA28gamma-dependent pathway in insulin resistance induced by hepatitis C virus core protein. J Virol. 2007. 81:1727–1735.
28. Leandro G, Mangia A, Hui J, et al. HCV Meta-Analysis (on) Individual Patients' Data Study Group. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006. 130:1636–1642.
29. Hourigan LF, Macdonald GA, Purdie D, et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology. 1999. 29:1215–1219.
30. Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001. 33:1358–1364.
31. Hwang SJ, Luo JC, Chu CW, et al. Hepatic steatosis in chronic hepatitis C virus infection: prevalence and clinical correlation. J Gastroenterol Hepatol. 2001. 16:190–195.
32. Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004. 126:586–597.
33. Lefkowitch JH, Schiff ER, Davis GL, et al. The Hepatitis Interventional Therapy Group. Pathological diagnosis of chronic hepatitis C: a multicenter comparative study with chronic hepatitis B. Gastroenterology. 1993. 104:595–603.
34. Björnsson E, Angulo P. Hepatitis C and steatosis. Arch Med Res. 2007. 38:621–627.
35. Kawaguchi T, Yamagishi S, Sata M. Branched-chain amino acids and pigment epithelium-derived factor: novel therapeutic agents for hepatitis c virus-associated insulin resistance. Curr Med Chem. 2009. 16:4843–4857.
36. Chitturi S, Abeygunasekera S, Farrell GC, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002. 35:373–379.
37. Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001. 96:2957–2961.
38. Mihm S, Fayyazi A, Hartmann H, Ramadori G. Analysis of histopathological manifestations of chronic hepatitis C virus infection with respect to virus genotype. Hepatology. 1997. 25:735–739.
39. Rubbia-Brandt L, Quadri R, Abid K, et al. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000. 33:106–115.
40. Hui JM, Kench J, Farrell GC, et al. Genotype-specific mechanisms for hepatic steatosis in chronic hepatitis C infection. J Gastroenterol Hepatol. 2002. 17:873–881.
41. Bedossa P, Moucari R, Chelbi E, et al. Evidence for a role of nonalcoholic steatohepatitis in hepatitis C: a prospective study. Hepatology. 2007. 46:380–387.
42. Patton HM, Patel K, Behling C, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004. 40:484–490.
43. Cua IH, Hui JM, Kench JG, George J. Genotype-specific interactions of insulin resistance, steatosis, and fibrosis in chronic hepatitis C. Hepatology. 2008. 48:723–731.
44. Zaitoun AM, Al Mardini H, Awad S, Ukabam S, Makadisi S, Record CO. Quantitative assessment of fibrosis and steatosis in liver biopsies from patients with chronic hepatitis C. J Clin Pathol. 2001. 54:461–465.
45. Henke JI, Goergen D, Zheng J, et al. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008. 27:3300–3310.
46. McPherson S, Jonsson JR, Barrie HD, O'Rourke P, Clouston AD, Powell EE. Investigation of the role of SREBP-1c in the pathogenesis of HCV-related steatosis. J Hepatol. 2008. 49:1046–1054.
47. Tsutsumi T, Suzuki T, Shimoike T, et al. Interaction of hepatitis C virus core protein with retinoid X receptor alpha modulates its transcriptional activity. Hepatology. 2002. 35:937–946.
48. Su AI, Pezacki JP, Wodicka L, et al. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002. 99:15669–15674.
49. Jackel-Cram C, Babiuk LA, Liu Q. Up-regulation of fatty acid synthase promoter by hepatitis C virus core protein: genotype-3a core has a stronger effect than genotype-1b core. J Hepatol. 2007. 46:999–1008.
50. Waris G, Felmlee DJ, Negro F, Siddiqui A. Hepatitis C virus induces proteolytic cleavage of sterol regulatory element binding proteins and stimulates their phosphorylation via oxidative stress. J Virol. 2007. 81:8122–8130.
51. Lerat H, Kammoun HL, Hainault I, et al. Hepatitis C virus proteins induce lipogenesis and defective triglyceride secretion in transgenic mice. J Biol Chem. 2009. 284:33466–33474.
52. Tarugi P, Averna M, Di Leo E, et al. Molecular diagnosis of hypobetalipoproteinemia: an ENID review. Atherosclerosis. 2007. 195:e19–e27.
53. Shaheen M, Echeverry D, Oblad MG, Montoya MI, Teklehaimanot S, Akhtar AJ. Hepatitis C, metabolic syndrome, and inflammatory markers: results from the Third National Health and Nutrition Examination Survey [NHANES III]. Diabetes Res Clin Pract. 2007. 75:320–326.
54. Serfaty L, Capeau J. Hepatitis C, insulin resistance and diabetes: clinical and pathogenic data. Liver Int. 2009. 29:Suppl 2. 13–25.
55. Nishida T, Tsuji S, Tsujii M, et al. Oral glucose tolerance test predicts prognosis of patients with liver cirrhosis. Am J Gastroenterol. 2006. 101:70–75.
56. Sigal SH, Stanca CM, Kontorinis N, Bodian C, Ryan E. Diabetes mellitus is associated with hepatic encephalopathy in patients with HCV cirrhosis. Am J Gastroenterol. 2006. 101:1490–1496.
57. Thuluvath PJ. Higher prevalence and severity of hepatic encephalopathy in patients with HCV cirrhosis and diabetes mellitus: is presence of autonomic neuropathy the missing part of the puzzle? Am J Gastroenterol. 2006. 101:2244–2246.
58. Cammà C, Petta S, Di Marco V, et al. Insulin resistance is a risk factor for esophageal varices in hepatitis C virus cirrhosis. Hepatology. 2009. 49:195–203.
59. Chu CJ, Lee SD, Hung TH, et al. Insulin resistance is a major determinant of sustained virological response in genotype 1 chronic hepatitis C patients receiving peginterferon alpha-2b plus ribavirin. Aliment Pharmacol Ther. 2009. 29:46–54.
60. Poustchi H, Negro F, Hui J, et al. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008. 48:28–34.
61. Romero-Gómez M, Del Mar Viloria M, Andrade RJ, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005. 128:636–641.
62. Dai CY, Huang JF, Hsieh MY, et al. Insulin resistance predicts response to peginterferon-alpha/ribavirin combination therapy in chronic hepatitis C patients. J Hepatol. 2009. 50:712–718.
63. Vlotides G, Sörensen AS, Kopp F, et al. SOCS-1 and SOCS-3 inhibit IFN-alpha-induced expression of the antiviral proteins 2,5-OAS and MxA. Biochem Biophys Res Commun. 2004. 320:1007–1014.
64. Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000. 6:77–86.
65. Del Campo JA, Romero-Gómez M. Steatosis and insulin resistance in hepatitis C: a way out for the virus? World J Gastroenterol. 2009. 15:5014–5019.
66. Chung WJ, Jang BK, Hwang JS, et al. PI3-kinase inhibition blocks interferon signaling in HCV infected hepatocytes. Hepatology. 2010. 52:Suppl 4. 827A.
67. Bressler BL, Guindi M, Tomlinson G, Heathcote J. High body mass index is an independent risk factor for nonresponse to antiviral treatment in chronic hepatitis C. Hepatology. 2003. 38:639–644.
68. Giannini E, Ceppa P, Testa R. Steatosis in chronic hepatitis C: can weight reduction improve therapeutic efficacy? J Hepatol. 2001. 35:432–433.
69. Banerjee D, Williams EV, Ilott J, Monypenny IJ, Webster DJ. Obesity predisposes to increased drainage following axillary node clearance: a prospective audit. Ann R Coll Surg Engl. 2001. 83:268–271.
70. Chen CL, Yang HI, Yang WS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008. 135:111–121.
71. Tazawa J, Maeda M, Nakagawa M, et al. Diabetes mellitus may be associated with hepatocarcinogenesis in patients with chronic hepatitis C. Dig Dis Sci. 2002. 47:710–715.
72. Ohata K, Hamasaki K, Toriyama K, et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003. 97:3036–3043.
73. Lawson DH, Gray JM, McKillop C, Clarke J, Lee FD, Patrick RS. Diabetes mellitus and primary hepatocellular carcinoma. Q J Med. 1986. 61:945–955.
74. Barker BE, Fanger H, Farnes P. Human mammary slices in organ culture. I. Method of culture and preliminary observations on the effect of insulin. Exp Cell Res. 1964. 35:437–448.
75. Colhoun HM. SDRN Epidemiology Group. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009. 52:1755–1765.
76. Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009. 52:1732–1744.
77. Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia. 2009. 52:1745–1754.
78. Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009. 52:1766–1777.
79. Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004. 127:1044–1050.
80. Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009. 137:482–488.
81. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010. 33:1674–1685.
82. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007. 132:2557–2576.
83. Wands J. Hepatocellular carcinoma and sex. N Engl J Med. 2007. 357:1974–1976.
84. Komura T, Mizukoshi E, Kita Y, et al. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol. 2007. 102:1939–1946.
85. Romero-Gómez M, Diago M, Andrade RJ, et al. Spanish Treatment of Resistance to Insulin in Hepatitis C Genotype 1 Group. Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology. 2009. 50:1702–1708.
86. Harrison SA. Liver disease in patients with diabetes mellitus. J Clin Gastroenterol. 2006. 40:68–76.
87. Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996. 334:574–579.
88. Shishido S, Koga H, Harada M, et al. Hydrogen peroxide overproduction in megamitochondria of troglitazone-treated human hepatocytes. Hepatology. 2003. 37:136–147.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download