Abstract
Purpose
We compared location of positive cores in biopsy and location of positive surgical margin (PSM) following radical prostatectomy.
Materials and Methods
This retrospective analysis included patients who were diagnosed as prostate cancer by standard 12-core transrectal ultrasonography guided prostate biopsy, and who have PSM after radical prostatectomy. After exclusion of number of biopsy cores <12, and lack of biopsy location data, 46 patients with PSM were identified. Locations of PSM in pathologic specimen were reported as 6 difference sites (apex, base and lateral in both sides). Discordance of biopsy result and PSM was defined when no positive cores in biopsy was identified at the location of PSM.
Results
Most common location of PSM were right apex (n=21) and left apex (n=15). Multiple PSM was reported in 21 specimens (45.7%). In 32 specimens (69.6%) with PSM, one or more concordant positive biopsy cores were identified, but 14 specimens (28%) had no concordant biopsy cores at PSM location. When discordant rate was separated by locations of PSM, right apex PSM had highest rate of discordant (38%). The discordant group had significantly lower prostate volume and lower number of positive cores in biopsy than concordant group.
Prostate cancer is the second most common cancer in male worldwide, which accounts for 14.5% of all cancer, according to GLOBOCAN 2012 from World Health Organization [1]. In United States and Europe, it is amounting to 25% of newly diagnosed cancer [2]. In Korea, prostate cancer is the fifth most common cancer in male [3]. Annual incidence of prostate cancer in Korea was 8.5 per 100,000 men in 1999 and it was increased to 27.0 per 100,000 men in 2012 [3]. The annual increase rate of prostate cancer incidence was 11.4%, which is the highest among cancers except thyroid cancer [3].
The standard test for pathologic confirm of prostate cancer is transrectal ultrasonography guided prostate biopsy (TRUS-Bx), and standard 12-core biopsy is used commonly as a method of TRUS-Bx [45]. The result of TRUS-Bx provide not only an information whether prostate cancer is present or not, but also data about the location and the extent of prostate cancer [6]. The detailed information is regarded as an important reference to decide treatment modalities such as active surveillance, radical prostatectomy and hormonal therapy.
In particular, the data about location and the extent from biopsy help surgeons to determine the extent of resection, when performing radical prostatectomy [4]. According to the biopsy result, physicians carefully decide whether to dissect closely or widely, and whether to save neurovascular bundle or not. The decision is very crucial in radical prostatectomy, because it affects result of positive surgical margin (PSM) [789].
However, TRUS-Bx is not a perfect method for evaluation of prostate cancer, because the small amount of sampled tissue cannot represent the whole prostate perfectly [101112131415]. For example, 10%-18% of patients with negative result at first TRUS-Bx were diagnosed as prostate cancer at second TRUS-Bx [1011]. Therefore, we could not exclude a possibility that prostate cancer exists in the locations around each cores that were confirmed as benign in TRUS-Bx [16171819]. This imperfection of TRUS-Bx concerns physician about presence of undetected cancer which can cause PSM at the location where neurovascular bundle saving is performed [1617].
However, there are few reports that compares concordance between the location of PSM and the location of positive cores in TRUS-Bx. In this study, we compared location of positive cores in biopsy and location of PSM following radical prostatectomy.
This study was approved by the Institutional Review Board of the Konkuk University Hospital (IRB No. KUH1130047). This retrospective study included 155 patents who were diagnosed as prostate cancer by transrectal ultrasonography guided prostate biopsy and received retropubic radical prostatectomy between August, 2005 and December, 2013. Among them, only patients with PSM at final pathologic report were included. After exclusion of lack of exact data about the location of biopsy and the location of positive cores, and the number of biopsy cores lower than 12, 50 patients were included in final analysis.
Standard 12-core biopsy was performed in all patients. The locations of standard 12-core biopsy were right lateral apex, right medial apex, right lateral mid, right medial mid, right lateral base, right medial base and symmetrical site of left lobe (Fig. 1). When suspicious hypoechoic lesion was present, 1 or 2 additional biopsies were performed and the location was regarded as the nearest core location of the 12 standard locations.
The location of PSM in our pathologic results was reported routinely as 10 separate locations as followings; right apex, right base, right lateral, right anterior, right posterior and symmetrical site of left lobe. At each location, presence of prostate cancer cells at margin was reported separately. When PSM was present at more than 2 locations, all locations of PSM were described.
To compare the location of PSM and the location of cancer positive cores, we defined geometrically concordant cores as Fig. 2. For example, when PSM was presented at right lateral side, we could assume that prostate cancer was located at least one of right lateral apex, right lateral mid and right lateral base, therefore one of the biopsy locations must be reported as positive cores theoretically. When we could identify at least one positive core among the locations, we regarded that the biopsy result is concordant. When we could not identify any of positive biopsy cores at the assumed locations, we regarded that the result is discordant. In case of anterior PSM and posterior surgical margin, we could not specify assumed location of cancer exactly at coronal section of biopsy mapping. Therefore, the judgment of concordance or discordance at these PSM locations was not evaluated, and 4 cases with PSM exclusively at anterior or posterior location were excluded in comparative analysis. When multiple locations of PSM were presented, we compared each site and regarded the result is concordant only when we could find concordant cores at every location of PSM (concordant group), and regarded that the result is discordant when one or more discordant result was presented at any PSM locations (discordant group). We evaluated the concordant rate according to PSM. Patient characteristics and tumor characteristics were compared between concordant group and discordant group.
All statistical analyses were performed using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). A comparative analysis between the two groups was performed using the independent t-test for parametric continuous variables and the Mann-Whitney U test for nonparametric continuous variables. Fisher exact test was used to compare categorical variables. To evaluate factors that influence on discordant result, binary logistic regression analysis was used. The reported p-values were two-sided, and a p-value of 0.05 was considered statistically significant.
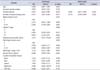
Mean age of 50 patients with PSM was 65.8±6.6 years. Baseline characteristics of patients were presented in Table 1. Mean prostate-specific antigen (PSA) of the patients was 22.9±38.1 ng/mL. Most of patients (n=48, 96%) received 12-core TRUS-Bx, and remained 2 patients received 12-core biopsy and 1 or 2 additional targeted biopsy. Mean number of positive cores were 5.7±3.6. All cases had no evidence of metastasis.
Most common location of PSM in pathologic specimen was right apex (n=21, 42%) followed by left apex (n=15, 30%), left base (n=11, 22%) and right base (n=10, 20%) (Table 2). In 29 specimens (58%) showed PSM at only one location, however 21 specimens (42%) identified to have PSM at two or more locations (Table 1).
On comparative analysis, one or more concordant positive biopsy cores were identified at every PSM locations in 32 specimens (69.6%) with PSM, but 14 specimens (30.4%) had no concordant biopsy cores at one of PSM locations (Table 2). When discordant rate was separated by locations of PSM, right apex PSM had highest rate of discordance (38%).
When we compared patient characteristics and tumor characteristics between concordant group and discordant group, age, PSA, biopsy Gleason score sum, pathologic Gleason score sum, pathologic T stage and percent tumor volume were not different between two groups. However, prostate volume was smaller, and number of cancer positive cores was lower in discordant group, compared to concordant group (Table 3). On multivariate analysis, number of cancer positive cores and prostate volume were independent factors that were related to discordant result (Table 4).
This study revealed that about one fourth of PSM occurred at location where prostate cancer is not detected in standard 12-core TRUS-Bx. This implies that prostate cancer existed at the location where cancer was not detected in TRUS-Bx. For the reason of missing, the undetected tumor might be so small that the cancer is located only between the biopsy cores. Inaccurate targeting also cause the negative results. In addition, out study showed that PSM occurred most at apex area commonly, and inconsistent result was also most common at the apex area.
The accuracy of TRUS-Bx was known to be only 70%-80% [1011]. As an example of inaccurate TRUS-Bx, prostate cancer was detected by repeat biopsy in 25%-30% patients who showed negative result at first TRUS-Bx [12131415]. Abraham et al. [10] reported that prostate cancer was detected in 63 patients (24.7%) among 255 men who underwent repeat biopsies. Another study also reported that second, third, and fourth repeat biopsy procedures yielded a diagnosis of prostate cancer in 18%, 17%, and 14% of patients, respectively [11]. Numao et al. [17] also compared result of simultaneous transrectal 12-core biopsy and transperineal 14-core biopsy, and showed that 21% of cancer was missed when transrectal 12-core biopsy was performed only, and the missed lesion were frequently located anteriorly.
False negative TRUS-Bx results also cause discordance with pathology of radical prostatectomy specimen. Huo et al. [18] compared the results of primary prostate biopsy with the radical prostatectomy, and revealed that the sensitivity for the detection of cancer in all biopsy zones was only 48%, which means that over half of cancer was missed in TRUS-Bx.
Another retrospective analysis showed that the accuracy of biopsy at apical location was lower than other site [19]. The positive predictive value of a positive apical core for identifying correct tumor location in the prostatectomy specimen was 71.1%, while absence of cancer in the apical biopsy had a negative predictive value of 75.5%. Sensitivity of detecting apical cancer was as low as 44.5%. This study supported our results. Our study showed that most common location of PSM in pathologic specimen was right apex (42%) and apex area had highest rate of discordance (38%). These results imply that physician should be cautious to avoid PSM at apex regardless biopsy result.
Concerning the inaccuracy of TRUS-Bx, it was needed to evaluate whether decision of surgical extent based on the information from TRUS-Bx for reducing the risk of PSM was reasonable or not. To evaluate the reasonability, a few studies investigated consistency of biopsy result and surgical margin of pathologic specimen [1620]. When biopsy result was divided to right and left, 77% of biopsy-suggested unilateral carcinoma was found to be bilateral disease on final histology following radical prostatectomy [16]. Furthermore, 24% of unilateral disease on biopsy showed PSM at contralateral biopsy-benign side [16]. Touma et al. [20] divided biopsy location as apex and base, and investigated whether the location of preoperative biopsy positive cores could identify patients at higher risk of a PSM and extraprostatic extension at radical retropubic prostatectomy [20]. In the study, a positive biopsy at the apex was not predictive of an apical PSM or extraprostatic extension, but a positive biopsy at the base was related to a basal PSM (4% vs. 11%, p=0.003) and EPE (32% vs. 9%, p<0.001) [20].
Our results is very similar to these results that apex is more inaccurate than base. Furthermore, none of previous study subdivided the location of PSM as like our study. We subdivided the location of PSM by 6 separate locations, and our result revealed that discordant result is most common in apex, following by lateral and base. Furthermore, our study showed that discordance occurred at right apex than left apex.
Because prostate cancer have relatively good prognosis than other malignancies, the aim of radical prostatectomy is not only control of cancers, but also preserving quality of life after treatment [21]. In point of extended aim, concept of pentafecta-which mean five goals of radical prostatectomy-was developed [422]. Pentafecta includes continence, potency, cancer control (no recurrence), absence of postoperative complication, and negative surgical margin. Among those, continence, potency and surgical margin are affected by the extent of resection and neurovascular bundle saving during radical prostatectomy [789]. Especially, neurovascular bundle saving could preserve potency and improve continence results, but it increases the risk of PSM [4]. Therefore, surgeons always decides carefully whether to perform neurovascular bundle saving or not, according to the result of biopsy. However considering this study results, the pathologic locations of TRUS-Bx are not always reliable, especially in apex. Therefore, surgeon must assumed that the cancer exist at all location of prostate, and dissect carefully even in biopsy negative site when performing neurovascular bundle saving.
There is no definite prognostic variable to predict the discordant result between biopsy and pathologic specimen. In this study, that small prostate volume and small number of cancer core are related to the discordant results. Theoretically, biopsy of small prostate has low risk of missing cancers [18]. However, discrimination of exact location in TRUS-Bx is more difficult in small prostate. In addition, targeting marginal area of prostate is also more difficult in small prostate. These reasons might increase the discordant rate. In addition, relationship between small number of cancer positive biopsy cores and discordance could be explained as a result of inaccurate biopsy and neurovascular bundle saving based on the negative biopsy results.
Retrospective nature of this study and small number of cases were our major limitations. Because of the retrospective study design, neurovascular bundle saving and extent of dissection were not controlled during radical prostatectomy. In addition, we could not analyze the influence of discordance on biochemical recurrence and other oncologic outcomes due to insufficient number of cases. Nonetheless, we thought this study is meaningful because this is first study that described the discordant rate of biopsy result and pathologic PSM at subdivided locations.
This study showed that more than a quarter of PSM occurred at location where tumor was not detected at biopsy and that apex PSM had highest rate of discordant. Although tumor was not identified in specific biopsy location, careful dissection to avoid PSM should be performed in every location, especially apex.
Figures and Tables
Fig. 2
Geometrically concordant biopsy cores according to each positive surgical margin location. Blue tetragon is the location of positive surgical margin, and red ellipse is concordant biopsy cores of each positive surgical margin.
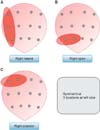
Table 1
Baseline characteristics of patients

Table 2
Location of positive surgical margin and concordance with biopsy

Table 3
Comparison of concordant group and discordant group

Table 4
Factors that influence on discordant result between location of positive surgical margin and location of positive biopsy cores
References
1. World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality, and prevalence worldwide in 2012 [Internet]. Lyon: International Agency for Research on Cancer;c2015. 2015 Jun 13. Availabl from:http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
2. Tao ZQ, Shi AM, Wang KX, Zhang WD. Epidemiology of prostate cancer: current status. Eur Rev Med Pharmacol Sci. 2015; 19:805–812.
3. National Cancer Center. Annual report of cancer statistics in Korea in 2012 [Internet]. Goyang: National Cancer Center;2015. 2015 Jun 29. Available from: http://ncc.re.kr/cancerStatsList.ncc?searchKey=total&searchValue=&pageNum=1.
4. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014; 65:124–137.
5. Iremashvili V, Pelaez L, Jorda M, Manoharan M, Arianayagam M, Rosenberg DL, et al. Prostate sampling by 12-core biopsy: comparison of the biopsy results with tumor location in prostatectomy specimens. Urology. 2012; 79:37–42.
6. Bjurlin MA, Wysock JS, Taneja SS. Optimization of prostate biopsy: review of technique and complications. Urol Clin North Am. 2014; 41:299–313.
7. Graefen M, Hammerer P, Michl U, Noldus J, Haese A, Henke RP, et al. Incidence of positive surgical margins after biopsy-selected nerve-sparing radical prostatectomy. Urology. 1998; 51:437–442.
8. Kessler TM, Burkhard FC, Studer UE. Nerve-sparing open radical retropubic prostatectomy. Eur Urol. 2007; 51:90–97.
9. Moore BM, Savdie R, PeBenito RA, Haynes AM, Matthews J, Delprado W, et al. The impact of nerve sparing on incidence and location of positive surgical margins in radical prostatectomy. BJU Int. 2012; 109:533–538.
10. Abraham NE, Mendhiratta N, Taneja SS. Patterns of repeat prostate biopsy in contemporary clinical practice. J Urol. 2015; 193:1178–1184.
11. Campos-Fernandes JL, Bastien L, Nicolaiew N, Robert G, Terry S, Vacherot F, et al. Prostate cancer detection rate in patients with repeated extended 21-sample needle biopsy. Eur Urol. 2009; 55:600–606.
12. Zaytoun OM, Stephenson AJ, Fareed K, El-Shafei A, Gao T, Levy D, et al. When serial prostate biopsy is recommended: most cancers detected are clinically insignificant. BJU Int. 2012; 110:987–992.
13. Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994; 151:1571–1574.
14. Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001; 166:1679–1683.
15. Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010; 362:1192–1202.
16. Connolly SS, O'Malley KJ, O'Brien A, Kelly DG, Mulvin DW, Quinlan DM. Can prostate biopsies predict suitability for nerve-sparing radical prostatectomy? Scand J Urol Nephrol. 2004; 38:216–220.
17. Numao N, Kawakami S, Sakura M, Yoshida S, Koga F, Saito K, et al. Characteristics and clinical significance of prostate cancers missed by initial transrectal 12-core biopsy. BJU Int. 2012; 109:665–671.
18. Huo AS, Hossack T, Symons JL, PeBenito R, Delprado WJ, Brenner P, et al. Accuracy of primary systematic template guided transperineal biopsy of the prostate for locating prostate cancer: a comparison with radical prostatectomy specimens. J Urol. 2012; 187:2044–2049.
19. Rogatsch H, Horninger W, Volgger H, Bartsch G, Mikuz G, Mairinger T. Radical prostatectomy: the value of preoperative, individually labeled apical biopsies. J Urol. 2000; 164(3 Pt 1):754–757.
20. Touma NJ, Chin JL, Bella T, Sener A, Izawa JI. Location of a positive biopsy as a predictor of surgical margin status and extraprostatic disease in radical prostatectomy. BJU Int. 2006; 97:259–262.
21. Eastham JA, Scardino PT, Kattan MW. Predicting an optimal outcome after radical prostatectomy: the trifecta nomogram. J Urol. 2008; 179:2207–2210.
22. Patel VR, Sivaraman A, Coelho RF, Chauhan S, Palmer KJ, Orvieto MA, et al. Pentafecta: a new concept for reporting outcomes of robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2011; 59:702–707.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download