Abstract
Primary small cell carcinoma of the prostate is a rare and very aggressive disease with a poor prognosis, even in its localized form. We managed a case of primary small cell carcinoma of the prostate. The patient was treated with robot-assisted laparoscopic radical prostatectomy and adjuvant chemotherapy. Herein we report this first case of robot-assisted laparoscopic radical prostatectomy performed in a patient with primary small cell carcinoma of the prostate.
Primary small cell carcinoma of the prostate is very rare, accounting for less than 2% of all prostate cancer cases [1]. It shows a very poor prognosis, and small cell cancer is confirmed in approximately 10% to 20% of deaths after the treatment of prostate adenocarcinoma [2]. The average age of onset has been reported to be 66 to 69 years; however, the range is broad from 24 to 92 years of age [1,2]. About half of the patients with primary small cell carcinoma of the prostate complain of symptoms due to bladder outlet obstruction, and one third of them complain of weight loss, anorexia, neurological symptoms, flank pain, and back pain [3]. The diagnosis is confirmed by a positive response to immunoperoxidase staining and neurosecretory granules visible by electron microscope.
We treated a patient surgically by robot-assisted laparoscopic prostatectomy after a diagnosis of primary small cell prostate cancer by transurethral resection of the prostate (TURP) and prostate biopsy due to acute urinary retention. Here we report this case together with a review of the literature.
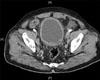
A 66-year-old male patient visited the urology clinic in another hospital because of acute urinary retention. His medical history included hypertension without any specific family history. His prostate-specific antigen (PSA) level and prostate volume as measured at the initial visit were 1.19 mg/ml (TandemR-R PSA immunoradiometric assay) and 53 g, respectively. The hard prostate was palpated by digital rectal examination, without any palpable masses, and there were no abnormal findings except for a white blood cell (WBC) count of 3-5/high power field (HPF) and a red blood cell (RBC) count of 3-5/HPF in the urine analysis. No abnormal findings were reported for the complete blood cell count, biochemistry indexes, and simple radiologic exam. In May 2009, TURP was performed on this patient, and abdominal pelvic computed tomography (CT) (Fig. 1), chest CT, and whole-body bone scan were also performed because the pathology results indicated small cell cancer. In the abdominal pelvic CT, multiple lymphadenopathies that were assumed to be metastatic lesions were observed near the bilateral iliac chain. No metastatic lesions were found on the chest CT or whole-body bone scan. To treat the primary small cell carcinoma of the prostate, the patient was admitted for robot-assisted laparoscopic radical prostatectomy with adjuvant chemotherapy after transfer to the urology clinic in our hospital.
We performed magnetic resonance imaging (MRI) in order to check the cancer stage. In the prostate MRI, the malignant lesion of the prostate was observed to invade the right capsule and seminal vesicles (Fig. 2). In July 2009, the patient underwent robot-assisted laparoscopic radical prostatectomy and extended pelvic lymph node dissection; the bilateral nerves were not saved. The total operative time was 240 minutes, and the amount of blood loss was 250 cc. The drainage tube was removed on day 3 after surgery, and the patient was discharged without any postoperative complications on day 4. The pathologic results showed small cell carcinoma invading the capsule of the prostate (Fig. 3, 4) and also metastasis of carcinoma in the right external iliac lymph nodes and right obturator lymph nodes. The weight of the prostate was 57 g, and the intraglandular tumor volume was 21 cc. The tumor involved both lobes, but the resection margin was not involved by the tumor. On postoperative day 7, the catheter was removed in the urology clinic. After brain MRI confirmed no metastatic lesions, adjuvant chemotherapy was started with VP-16 100 mg/m2 and cisplatin 80 mg/m2 on day 30. Adjuvant chemotherapy has been performed 8 times for 9 months since the operation. The multiple lymphadenopathies that had been observed before the surgery improved and there is currently no evidence of remnant disease.
Extrapulmonary small cell cancers are observed in the head and neck, skin, gastrointestinal system, cervix, and so on, but they are very rare, accounting for 0.1% to 0.4% of all malignant tumors [4] and approximately 5% of all small cell cancers. In the urological system, there have been case reports in the kidney, bladder, and prostate [5]. Small cell cancer of the prostate is known to occur as the result of changes in the argyrophil cells and argentaffin cells that exist in the normal prostate [6]. However, the hypothesis that small cell cancer of the prostate occurs with the differentiation of various protocells is now convincing [7,8], showing coexistence with prostate adenoma or past history of prostate adenoma and positive responses [9] in prostatic acid phosphatase and PSA. The proper treatment guidelines for small cell prostate cancer are unclear owing to its low prevalence and an insufficiency of studies compared with typical prostate adenocarcinoma. It has been reported that only radical prostatectomy shows therapeutic effects on early small cell cancer in the prostate [10]; however, adjuvant chemotherapy is generally used. Although the need for adjuvant radiotherapy is controversial, it should be considered if the pathologic report shows prostatic capsular invasion or resection marginal invasion.
In our patient, we performed robot-assisted laparoscopic radical prostatectomy and pelvic lymph node dissection. The patient did not undergo adjuvant radiotherapy but did receive adjuvant chemotherapy because no remaining cancer was reported by postoperative pathology, although it was reported that there had been metastasis in the right iliac lymph node and right obturator lymph node. At 9 months after surgery, the cancer had not recurred and the patient's urinary incontinence had improved compared with right after the surgery, with the use of ≤1 safety liner per day. A case of robot-assisted laparoscopic radical prostatectomy together with adjuvant chemotherapy to treat primary small cell cancer of the prostate has not been reported previously. Therefore, it may be valuable to study the clinical significance of this case through further follow-up monitoring.
Figures and Tables
FIG. 1
Abdominal pelvic computed tomography (CT) scan: lymphadenopathy and right pelvic dilatation, which were assumed to be metastatic lesions near both iliac arteries, were observed.
References
1. Yao JL, Madeb R, Bourne P, Lei J, Yang X, Tickoo S, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol. 2006. 30:705–712.
2. Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008. 32:65–71.
3. Abbas F, Civantos F, Benedetto P, Soloway MS. Small cell carcinoma of the bladder and prostate. Urology. 1995. 46:617–630.
4. Aygun C. Small cell carcinoma of the prostate: a case report and review of the literature. Md Med J. 1997. 46:353–356.
5. Freeman NJ, Doolittle C. Elevated prostate markers in metastatic small cell carcinoma of unknown primary. Cancer. 1991. 68:1118–1120.
6. Wenk RE, Bhagaven BS, Levy R, Miller D, Weisburger W. Ectopic ACTH, prostatic oat cell carcinoma, and marked hypernatremia. Cancer. 1977. 40:773–778.
7. Sule-Suso J, Brunt AM. Small cell carcinoma of the prostate. Br J Radiol. 1992. 65:726–728.
8. Morikawa T, Kobayashi S, Yamadori I, Okino T, Ohmori M, Kurose M. Small cell carcinoma of the prostate. A case report. Int Urol Nephrol. 1993. 25:379–384.
9. Ro JY, Tetu B, Ayala AG, Ordonez NG. Small cell carcinoma of the prostate. II. Immunohistochemical and electron microscopic studies of 18 cases. Cancer. 1987. 59:977–982.
10. Palmgren JS, Karavadia SS, Wakefield MR. Unusual and underappreciated: small cell carcinoma of the prostate. Semin Oncol. 2007. 34:22–29.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download