Abstract
Purpose
The National Cancer Institute (NCI)'s Hollow Fiber Assay (HFA) is currently used as an in vivo screening model to quantitatively define anticancer activity. To investigate the use of HFA in a bladder cancer model, we conducted in vitro and in vivo experiments with several anticancer drugs in nude mice.
Materials and Methods
The human bladder cancer cell lines (CRL2742, 253JP, SW1710, HTB9) were cultured both in vitro and in vivo in polyvinylidene fluoride (PVDF) hollow fibers. The fibers were implanted intraperitoneally (ip) and subcutaneously (sc) into female athymic nude mice (C57BL/6), and the mice were then treated with gemcitabine 120 mg/kg (bolus), cisplatin (3mg/kg), paclitaxel (15mg/kg) or vehicle only (control) for 4-consecutive days. After 6 days, the fibers were retrieved and the viable cell density was analyzed by MTT assay.
Results
The difference between in vitro and in vivo growth was not significant for the CRL2742, 253J-P and SW1710 cell lines; the difference between the ip and sc fibers was also not significant in the CRL2742, SW1710 and HTB9 cell lines. After drug treatment, the percent of growth inhibition revealed constant and effective anticancer activities for the 3 individual drugs.
REFERENCES
1.Hollingshead MG., Alley MC., Camalier RF., Abbott BJ., Mayo JG., Malspeis L, et al. In vivo cultivation of tumor cells in hollow fibers. Life Sci. 1995. 57:131–41.

2.Suggitt M., Bibby MC. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clin Cancer Res. 2005. 11:971–81.
3.Alami N., Paterson JS., Belanger S., Grieshaber CK., Leyland-Jones B. In vitro and in vivo activity of C1311 and paclitaxel in three cancer tumor models. AACR Meeting Abstracts. 2004. 2004:1069–70.
4.Hassan S., Dhar S., Sandstrom M., Arsenau D., Budnikova M., Lokot I, et al. Cytotoxic activity of a new paclitaxel formulation, Pacliex, in vitro and in vivo. Cancer Chemother Pharmacol. 2005. 55:47–54.

5.Sadar MD., Akopian VA., Beraldi E. Characterization of a new in vivo hollow fiber model for the study of progression of prostate cancer to androgen independence. Mol Cancer Ther. 2002. 1:629–37.
6.Leong CO., Suggitt M., Swaine DJ., Bibby MC., Stevens MF., Bradshaw TD. In vitro, in vivo, and in silico analyses of the antitumor activity of 2-(4-amino-3-methylphenyl)-5-fluoroben-zothiazoles. Mol Cancer Ther. 2004. 3:1565–75.
7.Hovstadius P., Lindhagen E., Hassan S., Nilsson K., Jernberg-Wiklund H., Nygren P, et al. Cytotoxic effect in vivo and in vitro of CHS 828 on human myeloma cell lines. Anticancer Drugs. 2004. 15:63–70.

8.Lee KH., Rhee KH. Correlative effect between in vivo hollow fiber assay and xenografts assay in drug screening. Cancer Res Treat. 2005. 37:190–200.

9.McMahon J., Schmid S., Weislow O., Stinson S., Camalier R., Gulakowski R, et al. Feasibility of cellular microencapsulation technology for evaluation of anti-human immunodeficiency virus drugs in vivo. J Natl Cancer Inst. 1990. 82:1761–5.

10.Wells RS., Campbell EW., Swartzendruber DE., Holland LM., Kraemer PM. Role of anchorage in the expression of tumorigenicity of untransformed mouse cell lines. J Natl Cancer Inst. 1982. 69:415–23.
11.Gorelik E., Ovejera A., Shoemaker R., Jarvis A., Alley M., Duff R, et al. Microencapsulated tumor assay: new short-term assay for in vivo evaluation of the effects of anticancer drugs on human tumor cell lines. Cancer Res. 1987. 47:5739–47.
12.Peterson JK., Houghton PJ. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer. 2004. 40:837–44.

13.Decker S., Hollingshead M., Bonomi CA., Carter JP., Sausville EA. The hollow fibre model in cancer drug screening: the NCI experience. Eur J Cancer. 2004. 40:821–6.
14.Havaleshko DM., Cho H., Conaway M., Owens CR., Hampton G., Lee JK, et al. Prediction of drug combination chemosensitivity in human bladder cancer. Mol Cancer Ther. 2007. 6:578–86.

15.Peters GJ., Bergman AM., Ruiz van Haperen VW., Veerman G., Kuiper CM., Braakhuis BJ. Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol. 1995. 22(4 Suppl 11):72–9.
16.Shipley LA., Brown TJ., Cornpropst JD., Hamilton M., Daniels WD., Culp HW. Metabolism and disposition of gemcitabine, and oncolytic deoxycytidine analog, in mice, rats, and dogs. Drug Metab Dispos. 1992. 20:849–55.
17.Braakhuis BJ., Ruiz van Haperen VW., Boven E., Veerman G., Peters GJ. Schedule-dependent antitumor effect of gemcitabine in in vivo model system. Semin Oncol. 1995. 22(4 Suppl 11):42–6.
18.van Moorsel CJ., Pinedo HM., Veerman G., Vermorken JB., Postmus PE., Peters GJ. Scheduling of gemcitabine and cisplatin in Lewis lung tumour bearing mice. Eur J Cancer. 1999. 35:808–14.

19.Casciari JJ., Hollingshead MG., Alley MC., Mayo JG., Malspeis L., Miyauchi S, et al. Growth and chemotherapeutic response of cells in a hollow-fiber in vitro solid tumor model. J Natl Cancer Inst. 1994. 86:1846–52.

20.Suggitt M., Swaine DJ., Pettit GR., Bibby MC. Characterization of the hollow fiber assay for the determination of microtubule disruption in vivo. Clin Cancer Res. 2004. 10:6677–85.

21.Bridges EM., Bibby MC., Burchill SA. The hollow fiber assay for drug responsiveness in the Ewing's sarcoma family of tumors. J Pediatr. 2006. 149:103–11.

22.Phillips RM., Pearce J., Loadman PM., Bibby MC., Cooper PA., Swaine DJ, et al. Angiogenesis in the hollow fiber tumor model influences drug delivery to tumor cells: implications for anticancer drug screening programs. Cancer Res. 1998. 58:5263–6.
23.Hollingshead MG., Bonomi CA., Borgel SD., Carter JP., Shoemaker R., Melillo G, et al. A potential role for imaging technology in anticancer efficacy evaluations. Eur J Cancer. 2004. 40:890–8.

24.Shnyder SD., Cooper PA., Scally AJ., Bibby MC. Reducing the cost of screening novel agents using the hollow fibre assay. Anticancer Res. 2006. 26:2049–52.
Fig. 1.
Implanted sc fibers (arrow) in a nude mouse (ip fibers are not shown). sc: subcutaneous, ip: intraperitoneal.
Fig. 2.
The differences between in vitro and in vivo (ip+sc) fibers in each of the bladder cancer cell lines. Three of four the cell lines (CRL2742, 253J-P, SW1710) except the HTB9 cell line, reveal no significant differences between the in vitro and in vivo fibers. The difference between the ip and sc fibers was also not significant, except for the 253J-P cell line. ip: intraperitoneal, sc: subcutaneous.
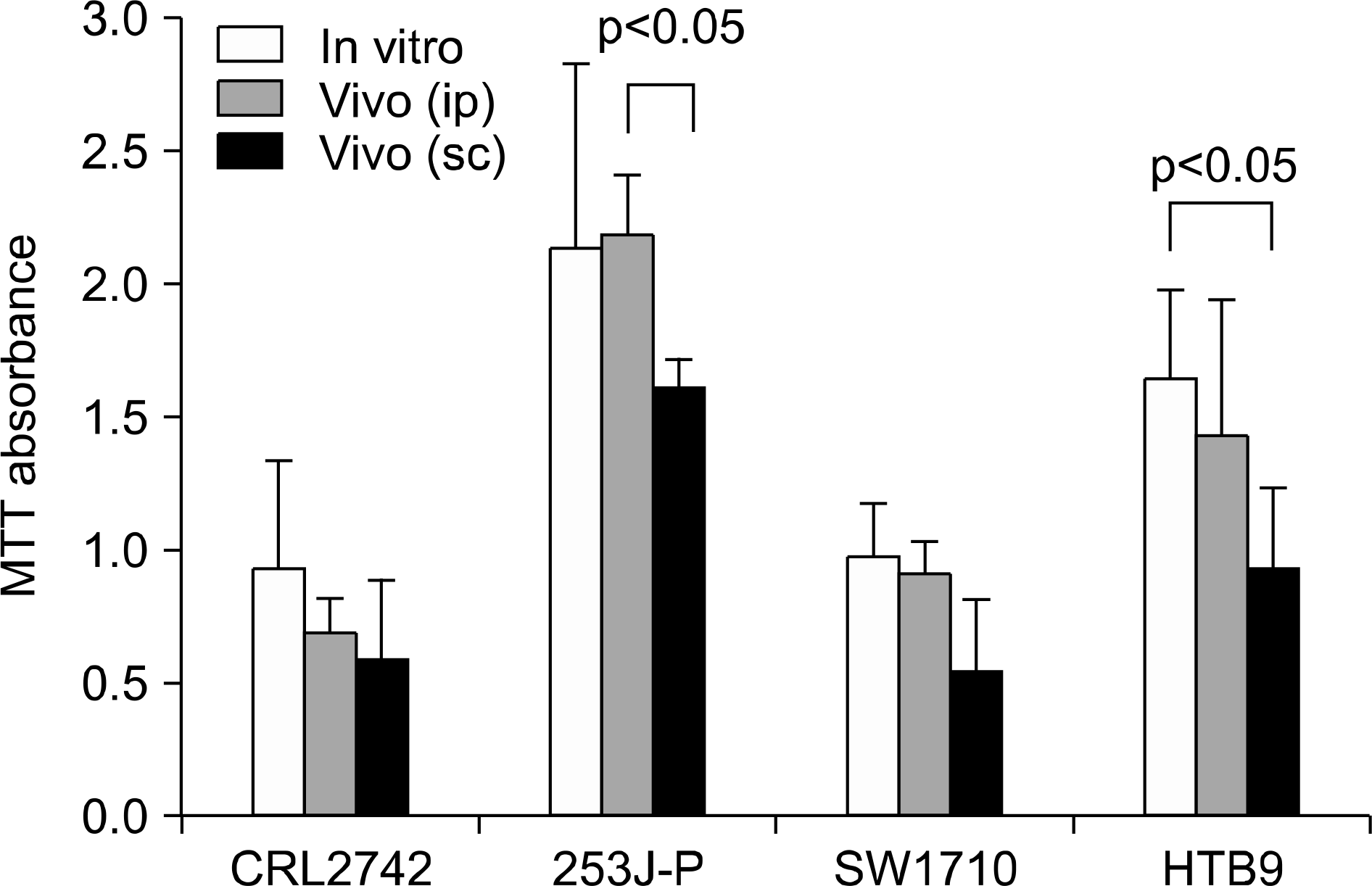
Fig. 3.
Percent (%) of growth inhibition (decease of MTT absorbance) after drug (cisplatin, paclitaxel, gemcitabine) injection for each of the cell lines. (A) % growth inhibition for the in vivo (ip+sc) fibers, (B) % growth inhibition for the in ip and sc fibers. ip: intraperitoneal, sc: subcutaneous, Cis: cisplatin, Tax: paclitaxel, Gem: gemcitabine.
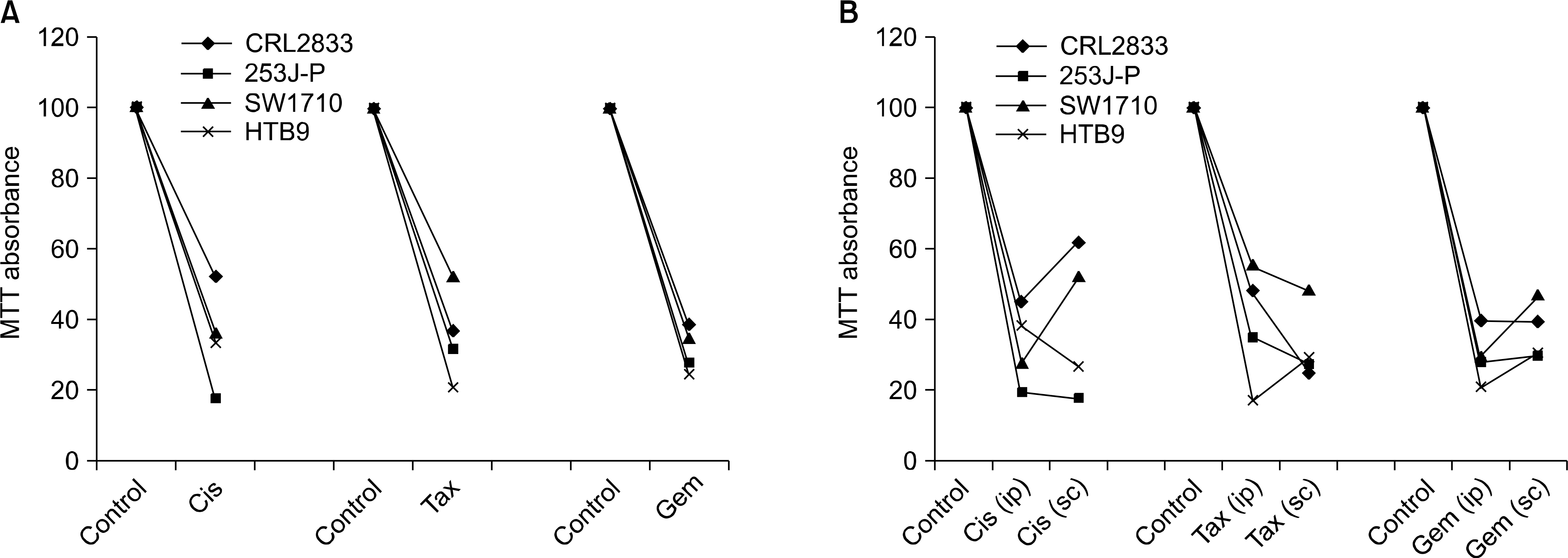
Table 1.
The MTT absorbance of the in vitro (upper) fibers (18 fibers per each cell line) and the in vivo (lower) intraperitoneal (ip) and subcutaneous (sc) fibers (8 fibers per each cell line)
Table 2.
The MTT absorbance after drug exposure (cisplatin 3mg/kg, paclitaxel 15mg/kg or gemcitabine 120mg/kg bolus) at 4-days (1-day for gemcitabine) after intraperitoneal injection. The control group received vehicle (normal saline) injection only. The individual growth inhibition % for each drug (lower)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download