Abstract
Purpose
We investigated whether periurethral injections of musclederived stem cells (MDSC) and chitosan/hydroapatite after denervation of rat's pudendal nerve could increase the leak point pressure over a long time period in a rat model of urinary incontinence.
Materials and Methods
Muscle-derived stem cells isolated from the gastrocnemius muscle of normal female rats were purified to obtain a myogenic population by using the preplate technique. The N group was the normal female rats, the D Group was the pudendal nerve transected group and the M Group was the MDSC/chitosan/hydroapatite composite gel injected group after pudendal nerve transection. The MDSC/chitosan/hydroapatite composite gel was injected into the proximal periurethral area. At 2 and 4 weeks, visually identified leak point pressure measurement was done with using the vertical tilt/intravesical pressure clamp model of urinary incontinence. The rats were then sacrificed and the periurethral tissues harvested for histological examination.
Results
The leak point pressure was significantly lower in the D group at each time compared with the N group, and the leak point pressure in the N and M groups were significantly higher than those in the D group at both 2 and 4 weeks. The persistence of MDSC over the period of study was verified by histological examination.
Figures and Tables
Fig. 1
Vertical tilt/intravesical pressure clamp model of urinary incontinence. Vertical tilt/intravesical pressure clamp model of urinary incontinence. (A) Acute spinal cord transection at the T9-T10 level was performed with the rat in a supine position and a transvesical catheter with a fire-flared tip (PE-90) was inserted into the dome of the bladder for measuring bladder filling and the pressure. (B) The intravesical pressure varied in 1-3cmH2O steps from zero upward until visual identification of the leak point (leak point pressure; LPP).
Fig. 2
Leak point pressure (LPP) at 2 weeks. The LPP in the D group was significantly lower at 1 month than the LPP at 1 month in the N and M groups. The LPP in the M group was significantly higher than that in the D group at 2 weeks (N: normal group, D: denervated group, M: denervated and muscle stem cell/chitosan/hydroapatite injected group). *: p<0.01 compared to N group, †: p<0.01 compared to D group.

Fig. 3
Leak point pressure (LPP) at 4 weeks. The LPP in the D group was significantly lower at 4 weeks than the LPP at 4 weeks in the N and M groups. The LPP in the M group was significantly higher than that in the denervation group at 4 weeks (N: normal group, D: denervated group, M: denervated and muscle stem cell/chitosan/hydroapatite injected group). *: p<0.01 compared to N group, †: p<0.01 compared to D group.

Fig. 4
Histology of the normal urethral sphincter of female rat. (A) Hematoxylin/eosin staining at 4 weeks. In the normal female rat urethral sphincter, a layer of striated muscle fibers (black arrow) encircles the smooth muscle fibers (red arrow). (B) MyHC (red) immunostaining of the specimen at 4 weeks post-treatment. In the normal female rat urethral sphincter, the striated muscle (white arrow) shows positive MyHC staining. (C) α-SMA (green) immunostaining of the specimen at 4 weeks post-treatment. The smooth muscle (red arrow) shows positive α-SMA staining, and the striated muscle (white arrow) shows blanks (A, B, C: ×100).
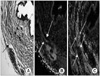
Fig. 5
Histology of the denervated urethral sphincter of female rat. (A) Hematoxylin/eosin staining at 4 weeks. The denervated proximal urethral sphincter also showed atrophic and thin circular striated fibers (black arrow). The smooth muscle is well preserved (red arrow). (B) MyHC immunostaining of specimen at 4 weeks post-treatment. The denervated proximal urethral sphincter, which is striated muscle (red arrow), shows negative MyHC staining. (C) α-SMA (green) immunostaining of the specimen at 4 weeks post-treatment. The smooth muscle (red arrow) shows positive α-SMA staining (A, B, C: ×100).
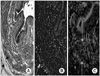
Fig. 6
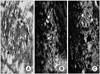
Histology of the MDSC/chitosan/hydroapatite injected urethral sphincter of female rat. (A) Hematoxylin/eosin staining at 4 weeks. The MDSC/chitosan/hydroapatite injection area (black arrow) is well preserved. (B) MyHC immunostaining of the specimen at 4 weeks post-treatment. The area where the MDSC was injected area is PKH26 positive (red) and the striated muscle (white arrow) shows positive MyHC staining. (C) α-SMA immunostaining of the specimen at 4 weeks post-treatment. Muscular bundles (white arrow) are observed to be positive for α-SMA staining (green) on the periphery of the polymer (A, B, C: ×100).
References
1. Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003. 14:31–37.
2. Yokoyama T, Huard J, Chancellor MB. Myoblast therapy for stress urinary incontinence and bladder dysfunction. World J Urol. 2000. 18:56–61.
3. Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002. 157:851–864.
4. Lee JY, Paik SY, Yuk SH, Lee JH, Ghil SH, Lee SS. The isolation and characterization of muscle derived stem cells from gastrocnemius muscle of rats using the modified preplate method. Korean J Urol. 2004. 45:1279–1284.
5. Lee JY, Paik SY, Yuk SH, Lee JH, Ghil SH, Lee SS. Long term effects of muscle-derived stem cells on leak point pressure and closing pressure in rats with transected pudendal nerves. Mol Cells. 2004. 18:309–313.
6. Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998. 142:1257–1267.
7. Berg S. Polytef augmentation urethroplasty. Correction of surgically incurable urinary incontinence by injection technique. Arch Surg. 1973. 107:379–381.
8. Shortliffe LM, Freiha FS, Kessler R, Stamey TA, Constantinou CE. Treatment of urinary incontinence by the periurethral implantation of glutaraldehyde cross-linked collagen. J Urol. 1989. 141:538–541.
9. Peeker R, Edlund C, Wennberg AL, Fall M. The treatment of sphincter incontinence with periurethral silicone implants (macroplastique). Scand J Urol Nephrol. 2002. 36:194–198.
10. Cervigni M, Tomiselli G, Perricone C, Panei M. Endoscopic treatment of sphincter insufficiency with autologous fat injection. Arch Ital Urol Androl. 1994. 66:219–224.
11. Emans PJ, Saralidze K, Knetsch ML, Gijbels MJ, Kuijer R, Koole LH. Development of new injectable bulking agents: biocompatibility of radiopaque polymeric microspheres studied in a mouse model. J Biomed Mater Res A. 2005. 73:430–436.
12. Henry DR, Barrett DM, Weiland TL, O'Conner MK, Malizia AA, Wien AJ. Particulate silicone for use in periurethral injections: local tissue effects and search for migration. J Urol. 1995. 153:2039–2043.
13. Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003. 62:958–963.
14. Yokoyama T, Yoshimura N, Dhir R, Zhuqing Qu, Fraser MO, Kumon H, et al. Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol. 2001. 165:271–276.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download