Abstract
Objective
We wanted to investigate the incidence of serious infections among the rheumatoid arthritis (RA) patients who were treated with tumor necrosis factor α (TNF-α) antagonists.
Methods
We enrolled the 175 RA patients who were treated with TNF-α antagonists for at least 3 months during February 2003 to July 2008, and these patients were in the SMART-b cohort of Kangnam St. Mary's hospital. Patients were prescribed infliximab, etanercept or adalimumab. The data was retrospectively collected.
Results
The incidence of serious infections among the RA patients treated with TNF-α was significantly increased according to the survival analysis, as compared with that of those patient treated with conventional DMARDs (p < 0.01). The most common serious infection was pneumonia. There was no significant difference in the incidence of serious infections among the three TNF-α antagonists used in this study (p=0.96). But the serious infections occurred more often in the patients who received more than 10 mg methotrexate (MTX) per week (p=0.02).
REFERENCES
1). Firestein GS. Immunologic mechanisms in the pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2005. 11:S39–44.

2). Botsios C. Safety of tumor necrosis factor and interleukin-1 blocking agents in rheumatic disease. Autoimmun Rev. 2005. 4:162–70.
4). Moreland LW., Schiff MH., Baumgartner SW., Tindall EA., Fleischmann RM., Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis. a randomized, Controlled Trial. Ann Intern Med. 1999. 130:478–86.
5). Genovese MC., Bathon JM., Martin RW., Fleischmann RM., Tesser JR., Schiff MH, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum. 2002. 46:1443–50.

6). Schneeweiss S., Setoguchi S., Weinblatt ME., Katz JN., Avorn J., Sax PE, et al. Anti-tumor necrosis factor aL therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheum. 2007. 56:1754–64.
7). St Clair EW. Infliximab treatment for rheumatic disease: clinical and radiological efficacy. Ann Rheum Dis. 2002. 61:ii67–ii69.

8). Doran MF., Crowson CS., Pond GR., O'Fallon WM., Gabriel SE. Frequency of Infection in patients with rheumatoid arthritis compared with controls: a population-based Study. Arthritis Rheum. 2002. 46:2287–93.

9). Ellerin T., Rubin RH., Weinblatt ME. Infections and anti-tumor necrosis factor alpha therapy. Arthritis Rheum. 2003. 48:3013–22.
10). Kroesen S., Widmer AF., Tyndall A., Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-alpha therapy. Rheumatology. 2003. 42:617–21.

11). Doran MF., Crowson CS., Pond GR., O'Fallon WM., Gabriel SE. Predictors of Infection in Rheumatoid Arthritis. Arthritis Rheum. 2002. 46:2294–300.

12). Listing J., Sttangfeld A., Kary S., Rau R., von Hinueber U., Stoyanova-Scholz M, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005. 52:3403–12.

13). Curtis JR., Patkar N., Xie A., Martin C., Allison JJ., Saag M, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor aL antagonists. Arthritis Rheum. 2007. 56:1125–33.
14). Bongartz T., Sutton AJ., Sweeting MJ. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systemic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006. 295:2275–85.
15). Wolfe F., Caplan L., Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia associations with prednisone, disease- modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum. 2006. 54:628–34.
16). Dixon WG., Watson K., Lunt M., Hyrich KL., Silman AJ., Symmons DPM. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving antitumor necrosis factor therapy results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006. 54:2368–76.

17). Dixon WG., Symmons DPM., Lunt M., Watson KD., Hyrich KL., Silman AJ. Serious infection following anti-tumor necrosis factor aL therapy in patients with rheumatoid arthritis lessons from interpreting data from observational studies. Arthritis Rheum. 2007. 56:2896–904.
18). Park HB., Kim KC., Park JH., Kang TY., Lee HS., Kim TH, et al. Association of reduced CD4 T cell responses specific to varicella zoster virus with high incidence of herpes zoster in patients with systemic lupus erythematosus. J Rheumatol. 2004. 31:2151–5.
19). Nagasawa K., Yamauchi Y., Tada Y., Kusaba T., Niho Y., Yoshikawa H. High incidence of herpes zoster in patients with systemic lupus erythematosus: an immunological analysis. Ann Rheum Dis. 1990. 49:630–3.

20). Wendling D., Streit G., Toussirot E., Prati C. Herpes zoster in patients taking TNF alpha antagonists for chronic inflammatory joint disease. Joint Bone Spine. 2008. 75:540–3.
21). Askling J., Fored CM., Brandt L., Baecklund E., Bertilsson L., CoUster L, et al. Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum. 2005. 52:1986–92.

22). Schiff MH., Burmester GR., Kent JD., Pangan AL., Kupper H., Fitzpatrick SB, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis. 2006. 65:889–94.

23). Centers for disease control and prevention (CDC). Tuberculosis associated with blocking agents against tumor necrosis factor-alpha-California, 2002-2003. MMWR Morb Mortal Wkly Rep. 2004. 53:683–6.
24). Hyrich KL., Silman AJ., Watson KD., Symmons DP. Anti-tumor necrosis factor aL therapy in rheumatoid arthritis: an update on safety. Ann Rheum Dis. 2004. 63:1538–43.
25). GutieArrez-MaciAas A., Lizarralde-Palacios E., MartiAnez-Odriozola P., Miguel-de la Villa F. Tuberculous peritonitis in a patient with rheumatoid arthritis treated with adalimumab. Clin Rheumatol. 2007. 26:452–3.

Fig. 1.
Incidence of serious infection in rheumatoid arthritis patients according to TNF- antagonists exposure (p.01). ∗TNF- antagonists group, conventional DMARDs group.
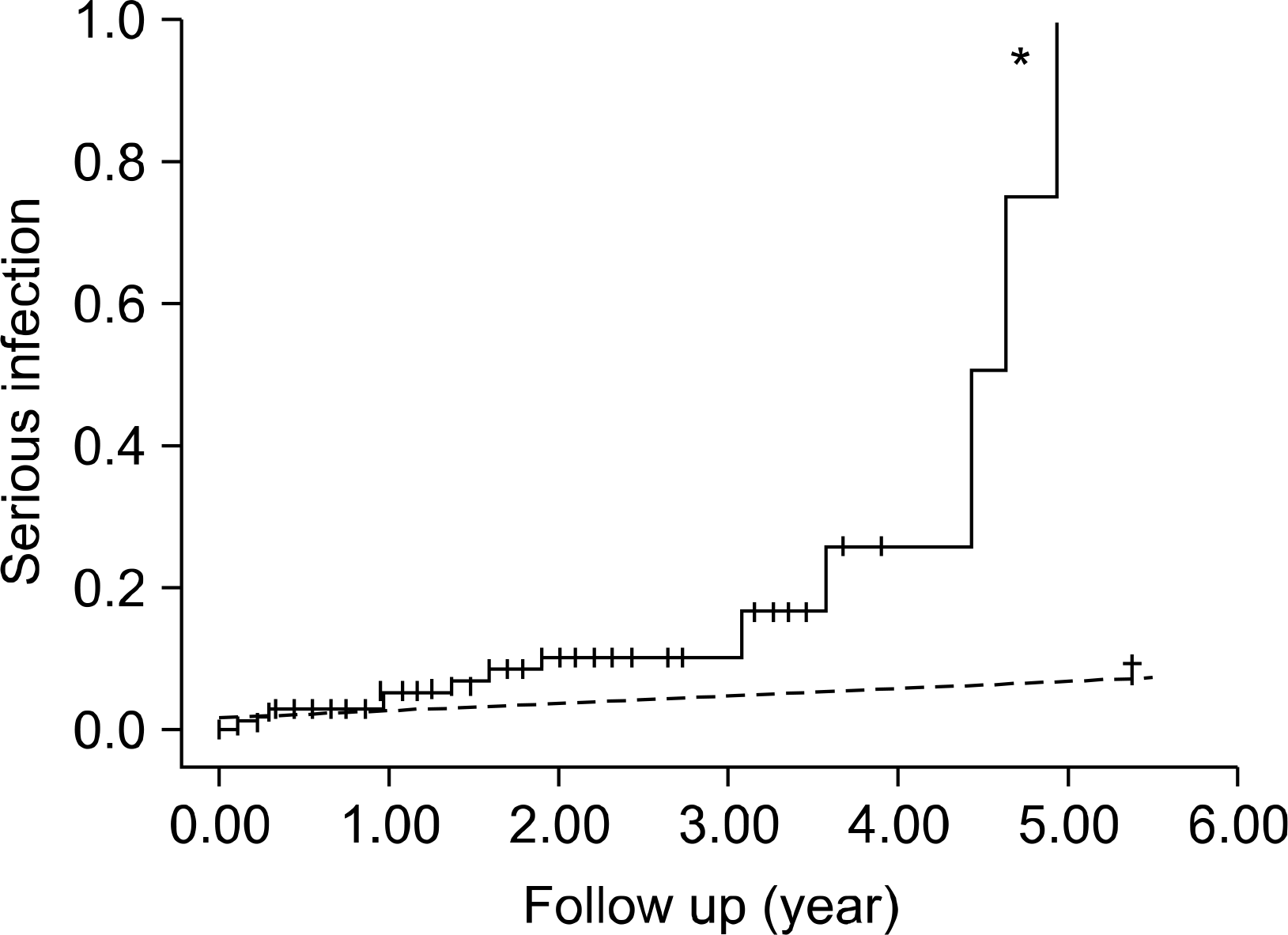
Fig. 2.
Incidence of serious infection among TNF- antagonists (p=0.96). ∗Inflixim ab, Etanercept, Adalimumab
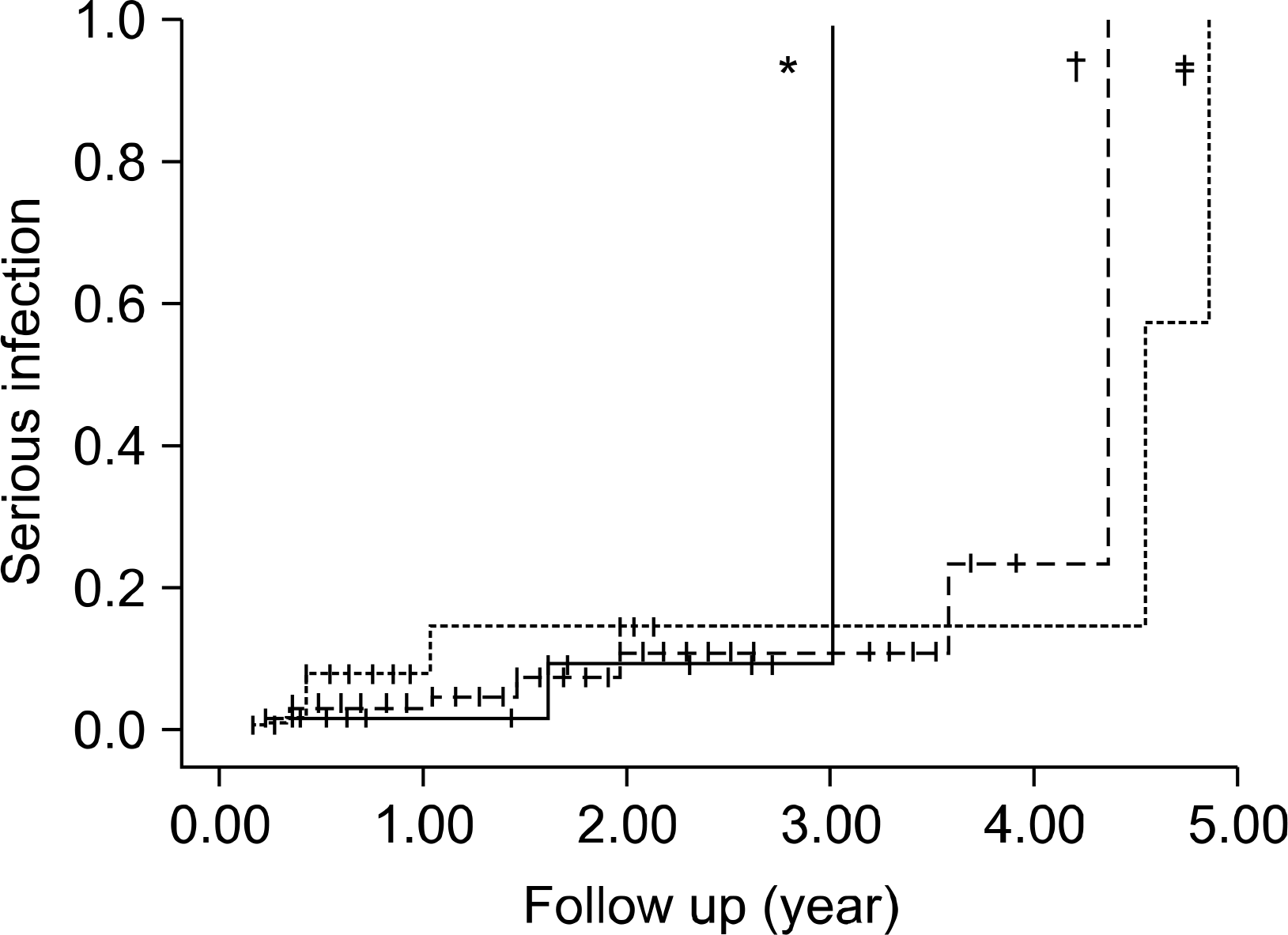
Table 1.
Baseline characteristics of enrolled RA patients
Table 2.
Incidence of serious infection in RA patients
Table 3.
Characteristics in RA patients exposed to TNF α antagonists according to serious infection




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download