Abstract
Background
It has been well established that daily injections of low dose parathyroid hormone (PTH) increase bone mass in animals and humans. However, the precise mechanisms by which PTH exerts its anabolic action on bone are incompletely understood. The canonical Wnt-β-catenin signaling pathway has recently been demonstrated to have an important role in bone cell function. In the present study, we have examined the interaction between the PTH and Wnt signaling pathways in mouse osteoblastic MC3T3-E1 cells.
Methods & Results
MC3T3-E1 cells were treated with 0.01?0.84 µM recombinant PTH. β-catenin expression was significantly increased after 30 minutes of exposure to PTH and reached a maximum 2.7 fold increase at 1 hr and expression then faded at 6 hrs. In addition, treatment with PTH increased nuclear accumulation of activated β-catenin; the ratio between the nuclear to cytoplasmic protein was more than three fold at 30 minutes and beyond. Moreover, PTH stimulated T-cell factor/lymphoid enhancer factor (TCF/LEF) reporter gene activity in MC3T3-E1 cells. Confocal microscopy revealed nuclear translocation of β-catenin by PTH as compared with a glycogen synthase kinase-3β (GSK-3β) inhibitor.
Figures and Tables
Fig. 1
Effect of PTH on levels of β-catenin in mouse osteoblastic cells. β-catenin expression was significantly increased after 30 minutes of exposure to PTH (10-8M) and maximized to 2.7 folds at 1 hr then faded at 6 hrs.

Fig. 2
Effects of PTH on nuclear or cytoplasmic active β-catenin expression. PTH (10-8M) increased nuclear accumulation of activated β-catenin; the ratio between nuclear to cytoplasmic protein were more than 3 folds at 30 minutes and thereafter

Fig. 3
Effects of PTH on intracellular translocation of β-catenin in MC3T3-E1 cells. (A) β-catenin expression (green) was weak without PTH. PTH (10-8M) treatment increases β-catenin expression in the cytoplasm as well as nuclei at 1 hr. β-catenin was mainly expressed in the nuclei after 6 hrs of PTH. LiCl (60 mM) increases β-catenin expression in the nuclei. (B) Hoechst staining of the same cells showing the nuclei (blue). (C) Confocal microscope images of the same field were taken and merged.
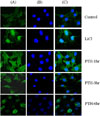
Fig. 4
Effects of PTH on the TCF/LEF reporter gene activity in mouse osteoblastic cells. PTH stimulates T-cell factor/ lymphoid enhancer factor (TCF/LEF) reporter gene activity in MC3T3-E1 cells. The changes in TCF/LEF reporter gene activity are expressed as fold change over control activity (no PTH treatment).

References
1. Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993. 14:690–709.
2. Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995. 136:3632–3638.
3. Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999. 104:439–446.
4. Onyia JE, Bidwell J, Herring J, Hullman J, Hock JM. In vivo, human parathyroid hormone fragment (hPTH 1-34) transiently stimulates immediate early response gene expression, but not proliferation, in trabecular bone cells of young rats. Bone. 1995. 17:479–484.
5. Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002. 157:303–314.
6. Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003. 18:960–974.
7. Pfeilschifter J, Laukhuf F, Műller-Beckmann B, Blum WF, Pfister T, Ziegler R. Parathyroid hormone increases the concentration of insulin-like growth factor-I and transforming growth factor beta 1 in rat bone
. J Clin Invest. 1995. 96:767–774.
8. Linkhart TA, Mohan S. Parathyroid hormone stimulates release of insulin-like growth factor-I (IGF-I) and IGF-II from neonatal mouse calvaria in organ culture. Endocrinology. 1989. 125:1484–1491.
9. Selvamurugan N, Pulumati MR, Tyson DR, Partridge NC. Parathyroid hormone regulation of the rat collagenase-3 promoter by protein kinase A-dependent transactivation of core binding factor alpha 1. J Biol Chem. 2000. 275:5037–5042.
10. Scott DK, Brakenhoff KD, Clohisy JC, Quinn CO, Partridge NC. Parathyroid hormone induces transcription of collagenase in rat osteoblastic cells by a mechanism utilizing cAMP and requiring protein synthesis. Mol Endocrinol. 1992. 6:2153–2159.
11. Potts JT, Bringhurst FR, Gardella T, Nussbaum S, Segre G, Kronenberg H. DeGroot LJ, editor. Parathyroid hormone: Physiology, chemistry, biosynthesis, secretion, metabolism and mode of action. Endocrinology. 2006. 5th ed. Philadelphia: WB Saunders Co;920–966.
12. Radeff JM, Singh ATK, Stern PH. Role of protein kinase A, phospholipase C and phospholipase D in parathyroid hormone receptor regulation of protein kinae C alpha and interleukin-6 in UMR-106 osteoblastic cells. Cell Signal. 2004. 16:105–114.
13. Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcriptional factor LEF-1. Nature. 1996. 382:638–642.
14. Krishnan V, Bryant HU, MacDougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006. 116:1202–1209.
15. Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001. 107:513–523.
16. Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor related protein 5. N Engl J Med. 2002. 346:1513–1521.
17. Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Mol Cell Biol. 2005. 25:4946–4955.
18. Bain G, Műller T, Wang X, YuPapkoff J. Activated β-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun. 2003. 301:84–91.
19. Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005. 132:49–60.
20. Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005. 8:727–738.
21. Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJS, Martin TJ, Gillespie MT, Onyia JE. Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem. 2005. 95:1178–1190.
22. Tobimatsu T, Kaji H, Sowa H, Naito J, Canaff L, Hendy GN, Sugimoto T, Chihara K. Parathyroid hormone increases β-catenin levels through smad3 in mouse osteoblastic cells. Endocrinology. 2006. 147:2583–2590.
23. Hock JM, Krishnan V, Onyia JE, Bidwell JP, Milas J, Stanislaus D. Osteoblast apoptosis and bone turnover. J Bone Miner Res. 2001. 16:975–984.
24. Hunter I, McGregor D, Robins SP. Caspase-dependent cleavage of cadherins and catenins during osteoblast apoptosis. J Bone Miner Res. 2001. 16:466–477.
25. Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005. 280:41342–41351.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download